IPFsym® a platform to support the development of effective treatments for IPF patients. IPFsym® can be used to predict efficacy for treatment modalities developed for IPF (and other lung diseases with custom modifications and additions). IPFsym has been utilized to evaluate a number of compounds within drug development, supporting clinical trial design optimization and clinical development decision making.

The IPFsym® QSP modeling software is a mechanistic, mathematical model of idiopathic pulmonary fibrosis (IPF).
The DILIsym Services division of SLP applies the IPFsym modeling software in proprietary services projects by performing simulations of compounds to evaluate and model their efficacy and other effects on key characteristics related to IPF. IPFsym is also available for in-house use through corporate software licensing.
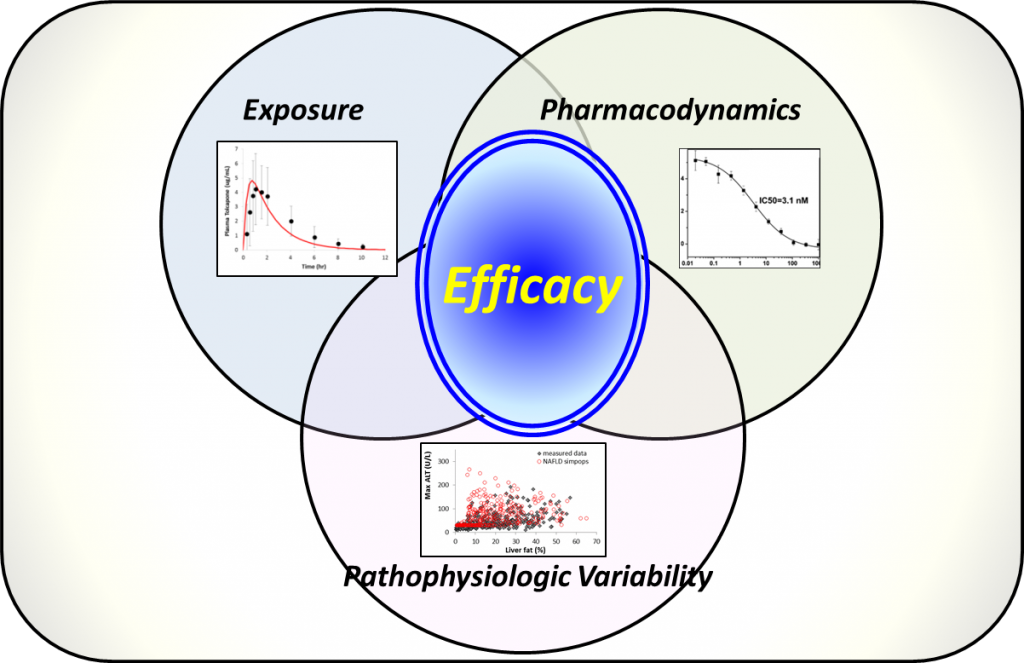
Idiopathic pulmonary fibrosis (IPF) is a fatal lung disease characterized by a progressive loss of lung function that often afflicts elderly patients. Genetic and environmental factors compromise the epithelial cells of the lung, leading to pulmonary fibrosis over time. DILIsym Services is leveraging our experience in modeling liver fibrosis in nonalcoholic fatty liver disease with NAFLDsym® to simulate lung fibrosis and other aspects of IPF. There is a substantial effort within the pharmaceutical industry to develop effective treatments for IPF, and we believe predictions with QSP modeling software like IPFsym will make the clinical development process more efficient, since QSP modeling has been demonstrated to accelerate clinical development. IPFsym employs the QSP modeling practices of combining predictions of compound exposure with the pharmacodynamic characteristics of a compound and how they can produce efficacy across a range of patients with pathophysiologic variability.
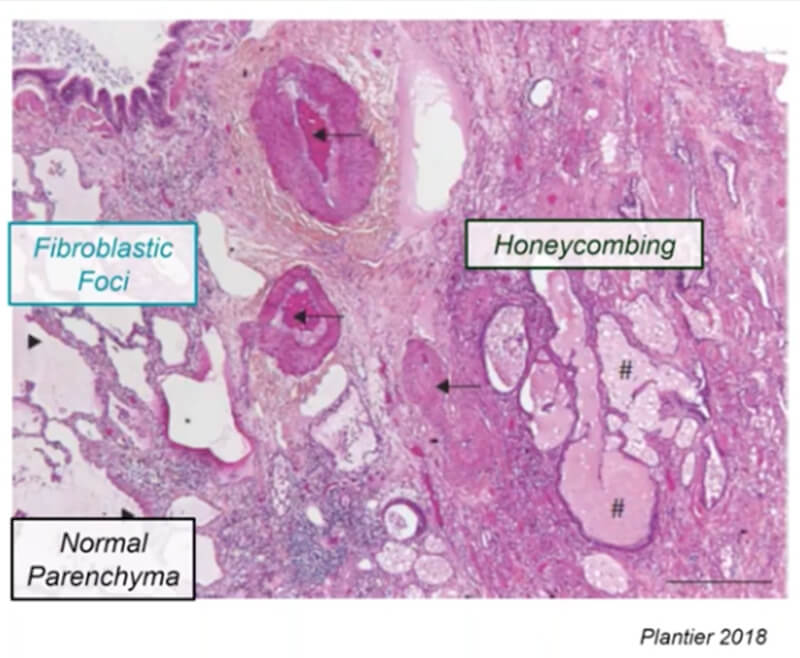
IPFsym version 1A includes key pathophysiologic mechanisms and clinical aspects of IPF, such as lung fibrosis, alveolar epithelial injury, inflammation, and lung function tests (e.g., forced vital capacity–FVC). Key regions of the lungs are represented, including the normal alveolar parenchyma, fibroblastic foci, and honeycombed regions comprised of extracellular matrix. IPFsym also includes the ability to simulate disease progression and inter-patient variability in pathophysiologic and clinical characteristics with SimPops®. It is useful for evaluating the efficacy of potential new drug candidates to treat IPF, including the powerful capability to evaluate combination treatment approaches.
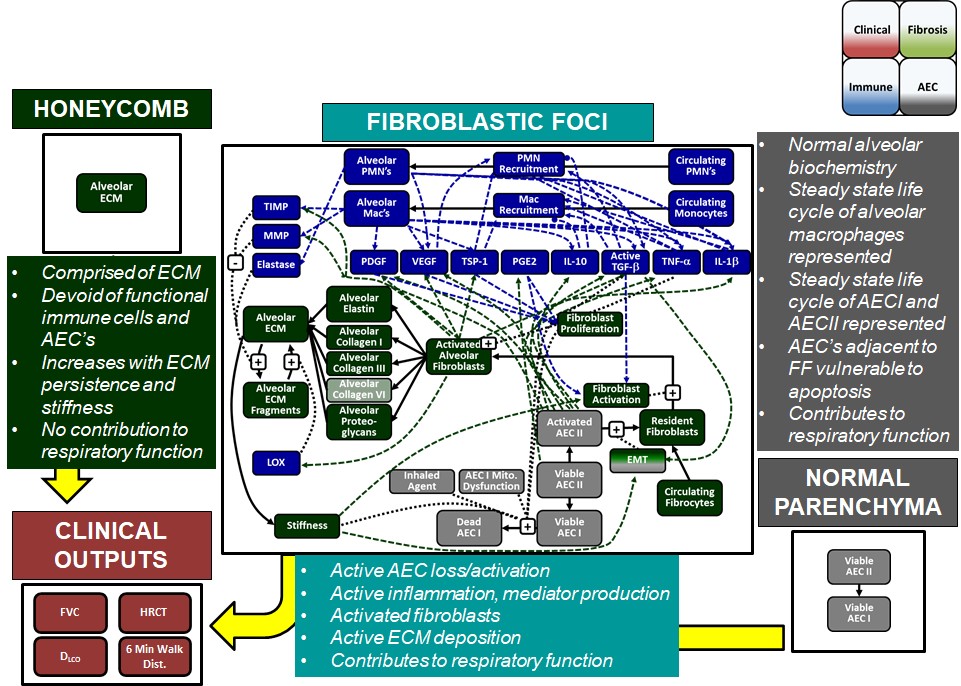
A number of key biomarkers or endpoints are incorporated into IPFsym v1A, such as:
- Forced vital capacity (FVC)
- Diffusing capacity of the lungs for carbon monoxide (DLCO)
- High resolution computed tomography (HRCT) of the lungs
- Fibroblastic foci volume
- Honeycombing volume
- 6 minute walk distance
- Collagen levels within the lungs
- Activated myofibroblasts
- Immune mediators
- TNF-alpha
- VEGF
- PDGF
- TGF-beta
- MMP-1
- IL-1-beta
- Immune cell infiltration
The heterogeneity within IPF, including the large variability in rate of disease progression, is a major challenge in the quest for new treatments. To this end, IPFsym v1A includes >500 simulated patients with varying stages of IPF. The simulated patients, or SimPops, can be used to optimize clinical trial protocols by determining favorable measurement frequencies and dosing levels, evaluating targets using key internal laboratory or mechanistic clinical data, testing combination treatment approaches across varying patient backgrounds, and comparing efficacy in different patient groups (e.g. stratification). The SimPops has been validated against a large backdrop of key data sets, such as those shown below for FVC, DLCO, and disease progression rates.
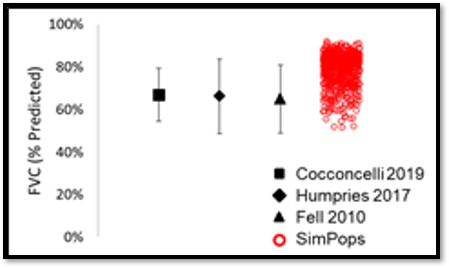
SimPops Validation with FVC Data
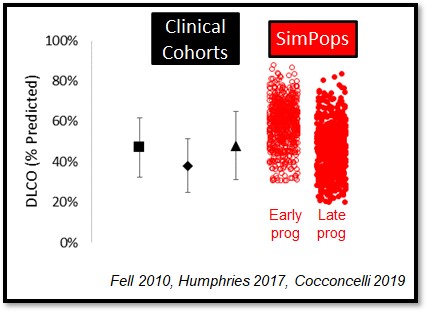
SimPops Validation with DLCO Data
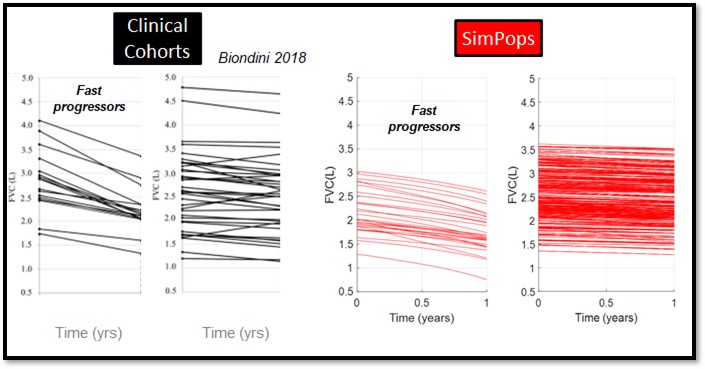
SimPops Validation with IPF Progression Rate Data
Validation of the various relationships within IPFsym v1A working in harmony has been done with the standards of care pirfenidone and nintedanib. Simulation results from SimPops with both drugs compared to placebo effects are shown below. The IPFsym simulations successfully recapitulate the drug effects relative to placebo.
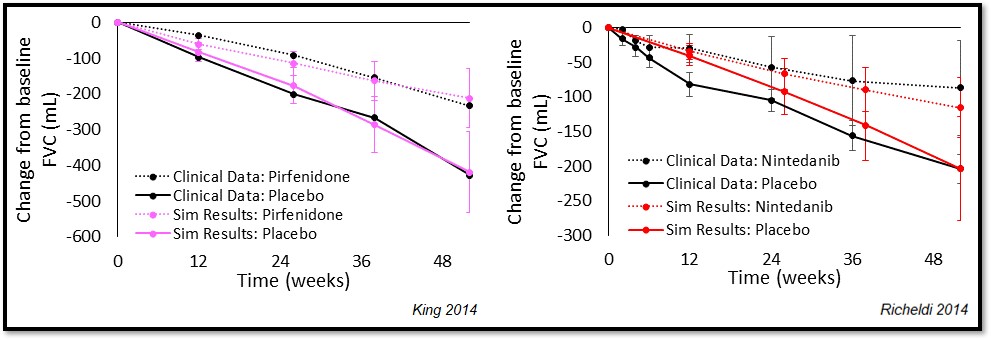
SimPops Validation with Pirfenidone and Nintedanib
IPFsym v1A is available for in-house use through corporate software licenses. The equations and parameter values are open for viewing and modification. MATLAB software is required for use and is available separately from MathWorks. Alternatively, let us partner with you on an IPFsym consulting project. Contact us today for more information on IPFsym license terms and pricing, as well as consulting options!
IPFsym® is computer software, namely, downloadable computer software for conducting simulations of compounds such as drugs or chemicals to evaluate and model their effects on the respiratory system, including the lungs.
To request a license for or evaluation of IPFsym®: https://www.simulations-plus.com/software-evaluation-request-form/