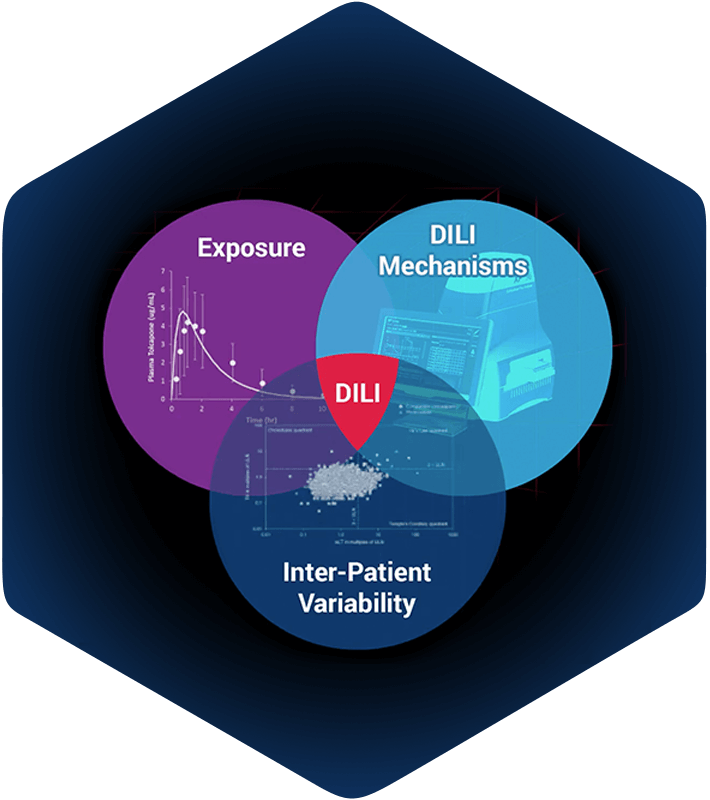
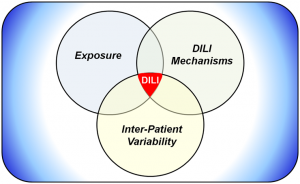
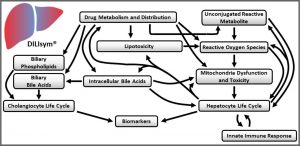
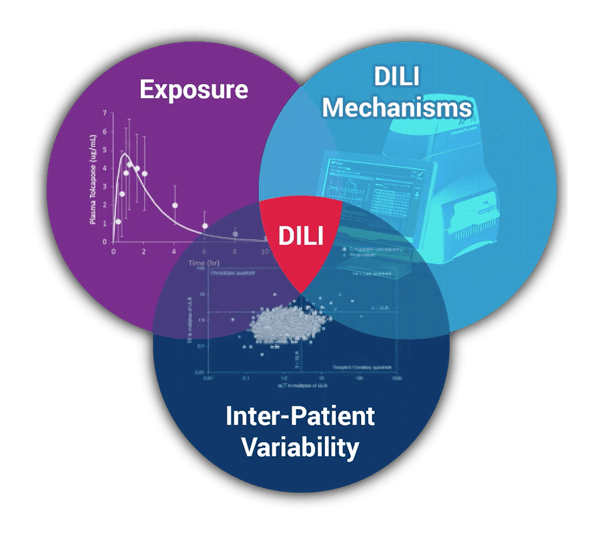
DILIsym is Quantitative Systems Toxicology (QST) software designed to be used during drug development to provide an indication of the potential drug-induced liver injury (DILI) hazard posed by individual molecules and/or to provide deeper insight into the mechanisms responsible for observed DILI responses at various stages of the development process.
- What are we providing with DILIsym?
- How is DILIsym being applied?
- What's new in DILIsym version X (DSX)?
DILIsym modeling software functions include:
- General population samples: The DILIsym software can be used to test compounds in simulated populations, termed SimPops. Simulated individuals within the SimPops express a wide‑range of response to the various exemplar compounds and can be characterized by extensive variability in their underlying biochemistry.
- DILIsym humans: A middle-out multi-scale representation of human physiology for assessing potential drug-induced liver injury (DILI) hazard in patients. Compound pharmacokinetic (PK) and pharmacodynamics (PD) information can be integrated to predict time profiles of liver enzymes (i.e., alanine aminotransferase, aspartate aminotransferase) and other clinical variables (e.g., bilirubin, prothrombin time, INR), as well as tissue properties (e.g., liver mass, GSH content). Alternate hypotheses regarding the downstream mechanisms of drug action can be investigated, including increased reactive oxygen/nitrogen species, ATP utilization, direct hepatocyte necrosis, and inhibition of bile acid transporters.
- DILIsym dogs: A middle-out multi-scale representation of Beagle dog physiology for assessing potential DILI hazard in dogs. Data from Beagle dogs were prioritized among the available datasets to maximize consistency in the representation.
- DILIsym rats: A middle-out multi-scale representation of Sprague-Dawley rat physiology for assessing potential DILI hazard in rats. Data from Sprague-Dawley rats were prioritized among the available datasets to maximize consistency in the representation. Compound pharmacokinetic and pharmacodynamics information can be integrated to predict time profiles as described above.
- DILIsym mice: A middle-out multi-scale representation of C57Bl/6 mouse physiology for assessing potential DILI hazard in mice. Data from C57Bl/6 mice were prioritized among the available datasets to maximize consistency in the representation. Compound pharmacokinetic and pharmacodynamics information can be integrated to predict time profiles as described above.
- Translational research: The ability to integrate your in vitro, small animal, and large animal compound data into a single platform facilitates translational research to better inform program advancement decisions, including experimental design, analyte selection, and timing of sampling.
DILIsym® is computer software, namely, computer software for modeling liver response to a drug or chemical; computer software for conducting simulations of drug-liver interactions; computer software for predicting pharmacokinetic, pharmacodynamic, and liver responses to a drug or chemical.