Every year, promising drug candidates move into clinical trials only to be flagged for potential drug-induced liver injury (DILI). Clinical DILI signals can result in delays, expensive additional studies and trials, or even in shelving the therapy altogether.
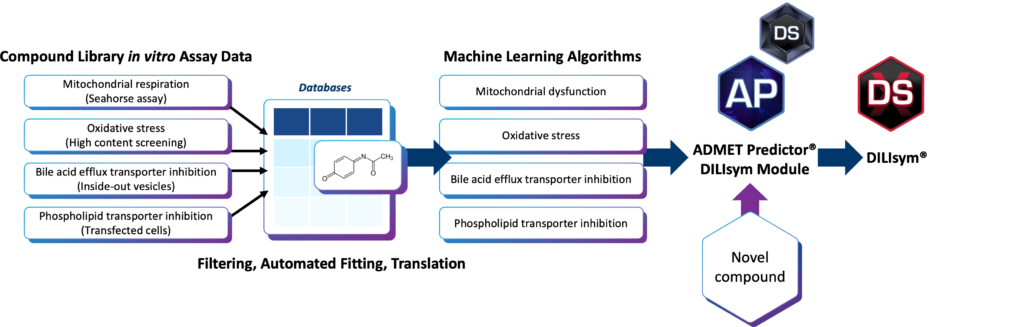
The DILIsym Inputs Module was designed to provide more insight into potential DILI risk during the early discovery phase and increase the likelihood of later clinical success.
The module generates outputs that inform inputs for DILIsym®, a quantitative systems toxicology (QST) modeling platform. Users will be able to conduct liver safety assessments, leveraging yes/no toxicity mechanism classifications and rank ordering of compound toxicity assessments with other in-class compounds—all without collecting and processing traditional in vitro toxicity assay data.