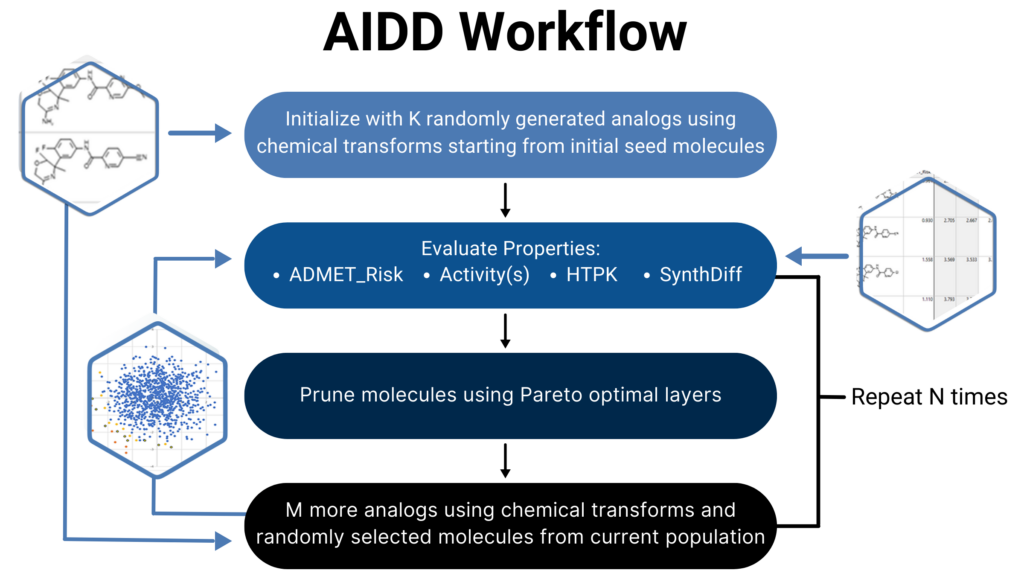
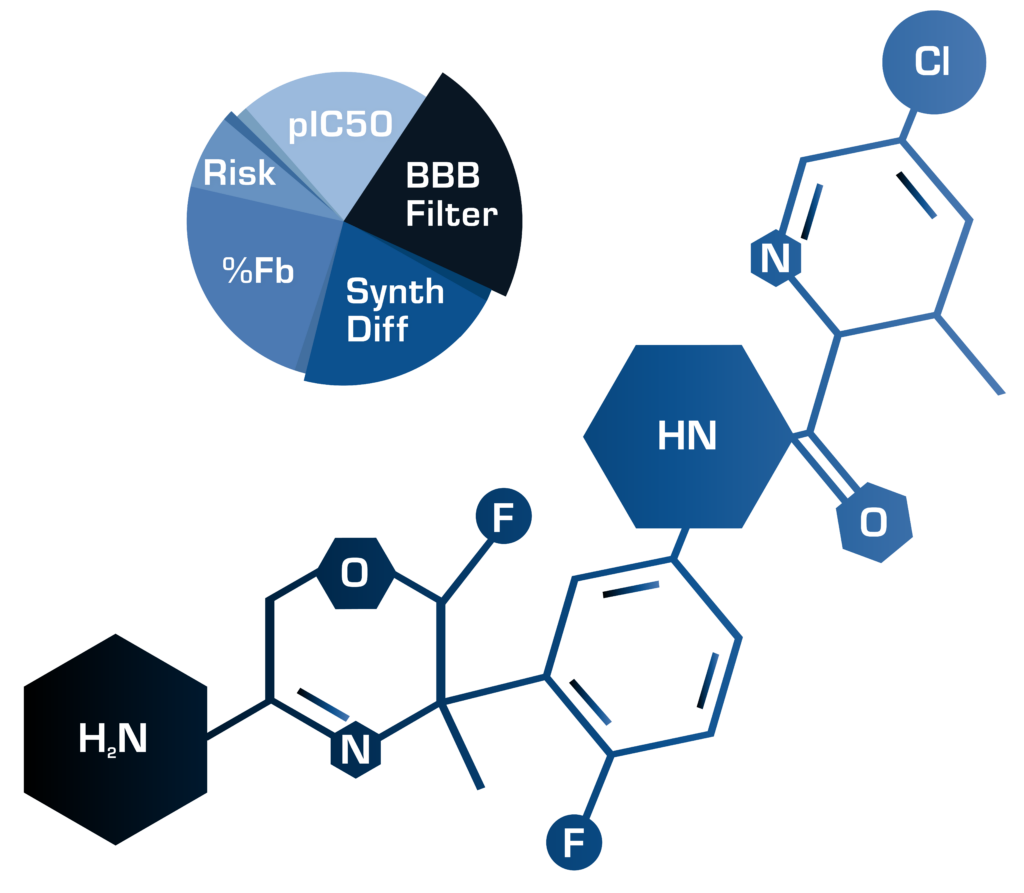
The original, semi-automated AIDD functionality was developed and validated in collaboration with GlaxoSmithKline and several other partners. The methodology and results can be read in the open access publication from Clark et al. in 2020.
More recently, a large pharmaceutical client was interested in designing new molecules that had an activity below 50 nanomolar for a specific target in the company’s therapeutic program of interest. We collaborated with them to develop and evaluate the AIDD module to meet their development needs.
Nearly 30 percent of the molecules designed by AIDD met their goal, with potencies as low as 16 nanomolar. Furthermore, rat and human microsomal clearance measurements for the compounds were accurately predicted, illustrating the potential for integrating physiologically-based pharmacokinetic (PBPK) modeling within the AIDD module and reliably applying systemic exposure considerations during lead compound selection. A manuscript reporting the details of this validation of the AIDD module is in preparation.
We are currently engaged in several internal projects and external collaborative research agreements to develop novel small molecules for a range of targets, the details of which will be reported in the coming months. If you would like to be part of our next success story, connect with one of our experts to discuss your drug discovery and development journey.