A Jump Start for Your Liver Safety Evaluation Program
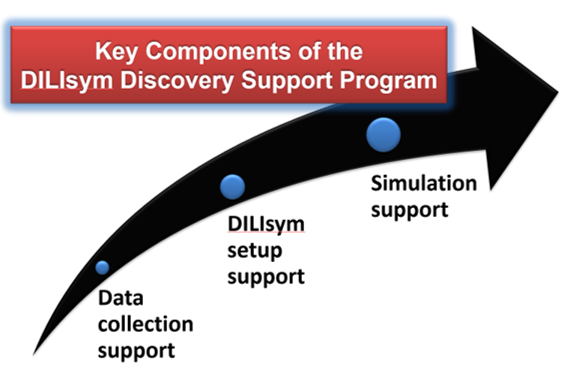 DILIsym Services, Inc., a Simulations Plus Company, offers a low cost solution for data acquisition and simulation set-up to support your in-house use of DILIsym. Our interdisciplinary team of experts can manage the experimental design and collection of in vitro data needed as DILIsym inputs, translate the in vitro data to toxicity parameter values, represent your compound in the DILIsym PBPK sub-model, and conduct initial simulations in a single simulated individual or a small group of simulated individuals (SimCohorts). Any or all of these options can be included in a DDSP project. With the results, you can then more efficiently use your time and resources with DILIsym to address the liver safety questions of interest (e.g., “Amongst my five potential candidates, which one has the best liver safety profile?” or “Which of my potential dosing protocols eliminates the liver signals we saw in clinical trials?”). This service is only offered to member companies of the DILI-sim Initiative. To receive a quote for DDSP services or membership, please Contact Us.
DILIsym Services, Inc., a Simulations Plus Company, offers a low cost solution for data acquisition and simulation set-up to support your in-house use of DILIsym. Our interdisciplinary team of experts can manage the experimental design and collection of in vitro data needed as DILIsym inputs, translate the in vitro data to toxicity parameter values, represent your compound in the DILIsym PBPK sub-model, and conduct initial simulations in a single simulated individual or a small group of simulated individuals (SimCohorts). Any or all of these options can be included in a DDSP project. With the results, you can then more efficiently use your time and resources with DILIsym to address the liver safety questions of interest (e.g., “Amongst my five potential candidates, which one has the best liver safety profile?” or “Which of my potential dosing protocols eliminates the liver signals we saw in clinical trials?”). This service is only offered to member companies of the DILI-sim Initiative. To receive a quote for DDSP services or membership, please Contact Us.
