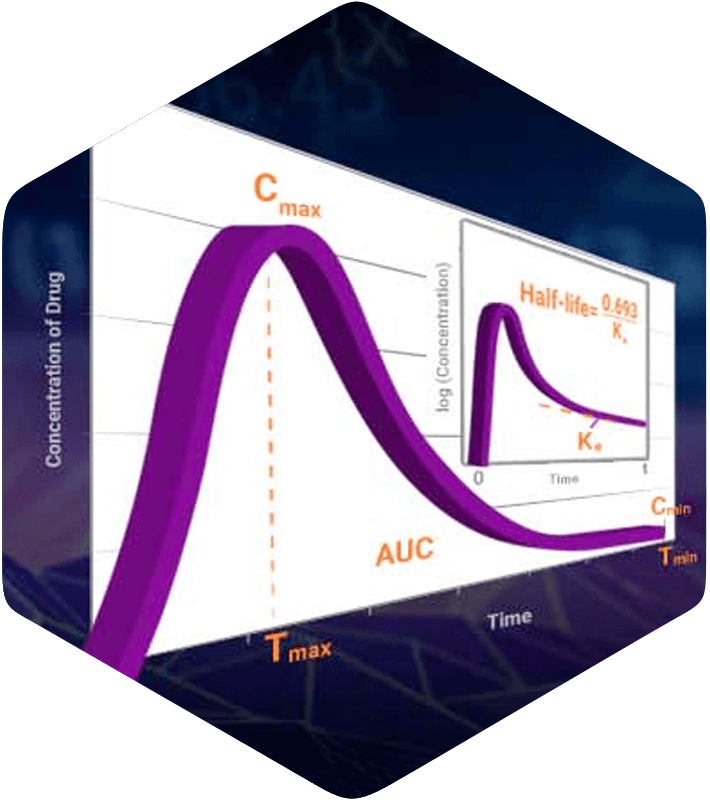
The success of your drug development program can hinge on the decisions you make at the earliest planning stages.
Our experienced clinical pharmacologists and pharmacometricians have worked on hundreds of projects with clients of all sizes, in multiple therapeutic areas and with different drug modalities, and are ready to advise and support your program from the design of preclinical studies all the way through your regulatory submission and post-marketing commitments.
Whether you have in-house experts who need support using state-of-the-art pharmacometrics software or you require clinical pharmacology and pharmacometrics modeling and strategy support, we’re ready to help.