Modeling is increasingly important for the speed and success of our drug development programs, but the learning curve to be effective can be high. It can take years to develop the expertise needed to build and refine reliable models.
The Monolix® application for population pharmacokinetic/pharmacodynamic (PK/PD) modeling comes with a large library of models and functionalities, and it keeps being expanded. The library comprises typical PK models, but also PD models, PK/PD models, and special models such as double-absorption PK, parent-metabolite, target-mediated drug disposition (TMDD), time-to-event (TTE), count, and tumor-growth inhibition models.
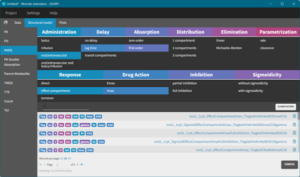
For instance, for a PK/PD dataset, a model is selected by just choosing the following features:
- Administration route
- Absorption delay
- Distribution (number of compartments)
- Elimination
- Parametrization (rate, clearance, or hybrid)
- Response type (direct, turnover, effect compartment)
- Drug action (Emax or Imax)
- Partial or full inhibition
- Sigmoidicity (Hill coefficient)
That’s it. Generally, no coding is required unless there are very special needs. For such special needs, users can start with a library model and edit it. All library functions come as full source code.
This simplicity allows junior modelers to quickly be effective and provides seasoned modelers valuable time to focus on answering questions about model assumptions and applications rather than de-bugging code or searching their archives for that unique control stream.
Here’s a typical workflow using the Monolix graphical user interface:
- Dataset loading
- Model selection from the library
- Visual inspection of data and model fits based on initial values. Initial values can be modified manually or with an auto-init function.
- Statistical model definition including random effects, covariates, and error structure using the graphical user interface.
- The model is ready to be run.
The simplicity of the software interface and the large model library allow users to…
- Efficiently develop models
- Substantially reduce the need for quality control of the code
- Improve the quality of analyses based on validated libraries
- Model with less experience or technical background
- Reduce time spent on code writing, freeing up time for trying alternatives, interpretation of results, and application of the model in simulations to address clinical questions
Making life easier for modelers is what Monolix is all about.
To see the simplicity of modeling with Monolix, watch our short demos and tutorials or schedule a demo.
