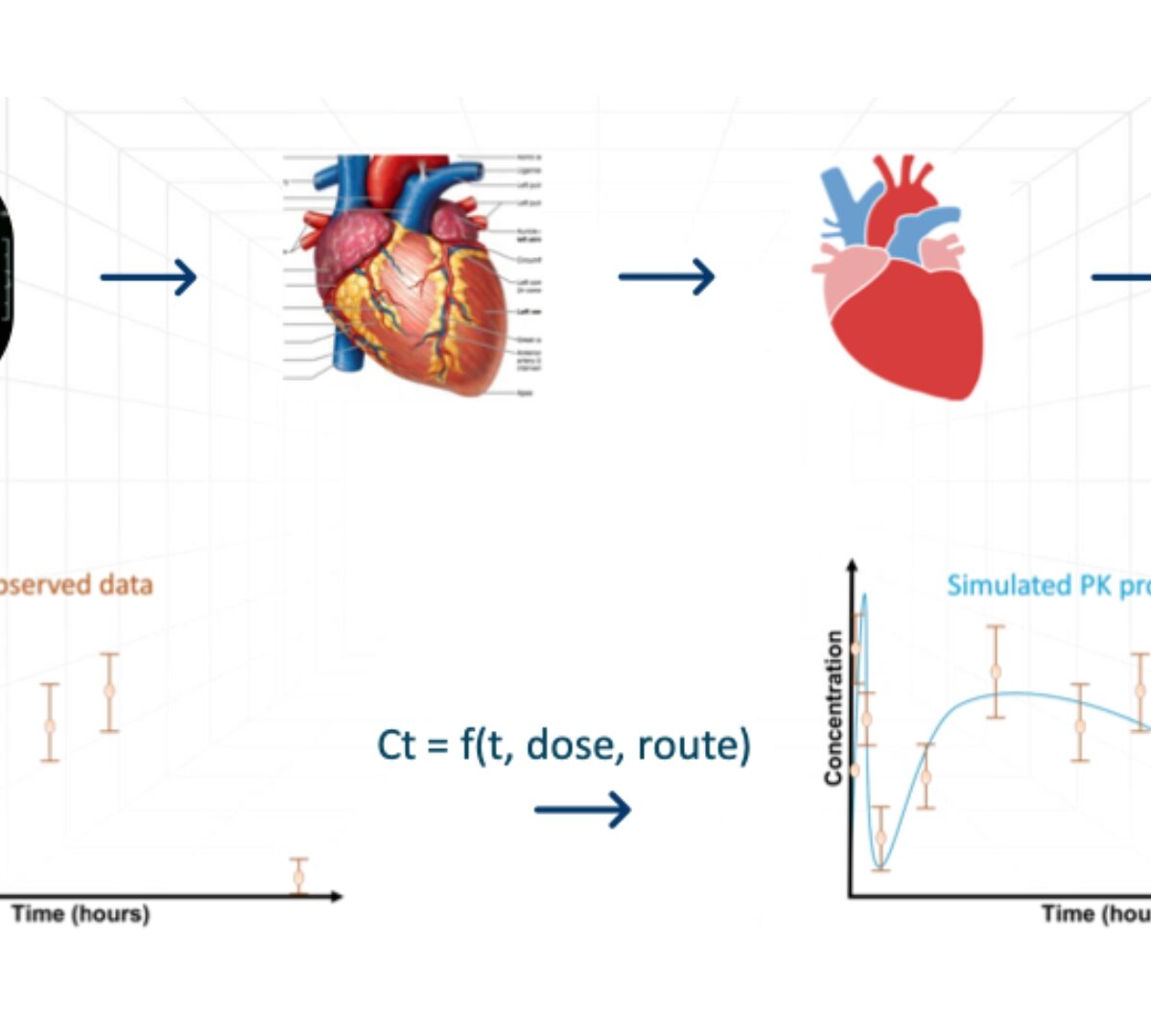
Application of Modeling and Simulation in Long-Acting Injectable Product Development
Discuss advances in mechanistic models to simulate in vitro and in vivobehavior of long acting injectables.
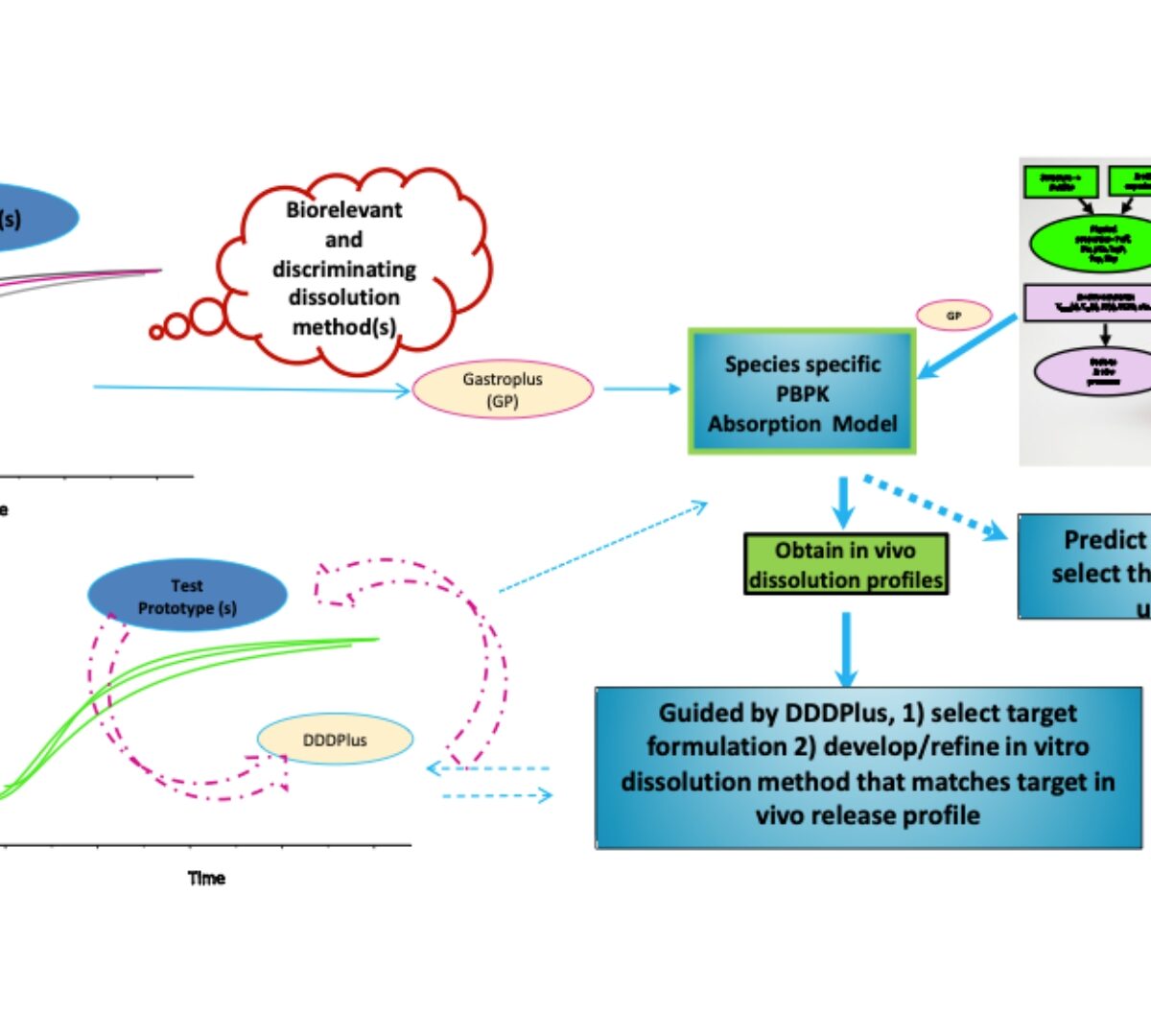
From Preclinical to Clinical Drug Product Development: A Path for Smooth Transition
It is challenging to establish a standard approach for moving from preclinical to clinical phases of development. Many hurdles exist during formulation selection and for extrapolation of doses from animals to humans; what may have worked well for one drug candidate may not be appropriate for others. This presentation will provide a general framework/strategy to follow when moving from preclinical to clinical studies (FIH) based on risk identification and mitigation focusing on the application of mechanistic modeling and simulation (PBPK).
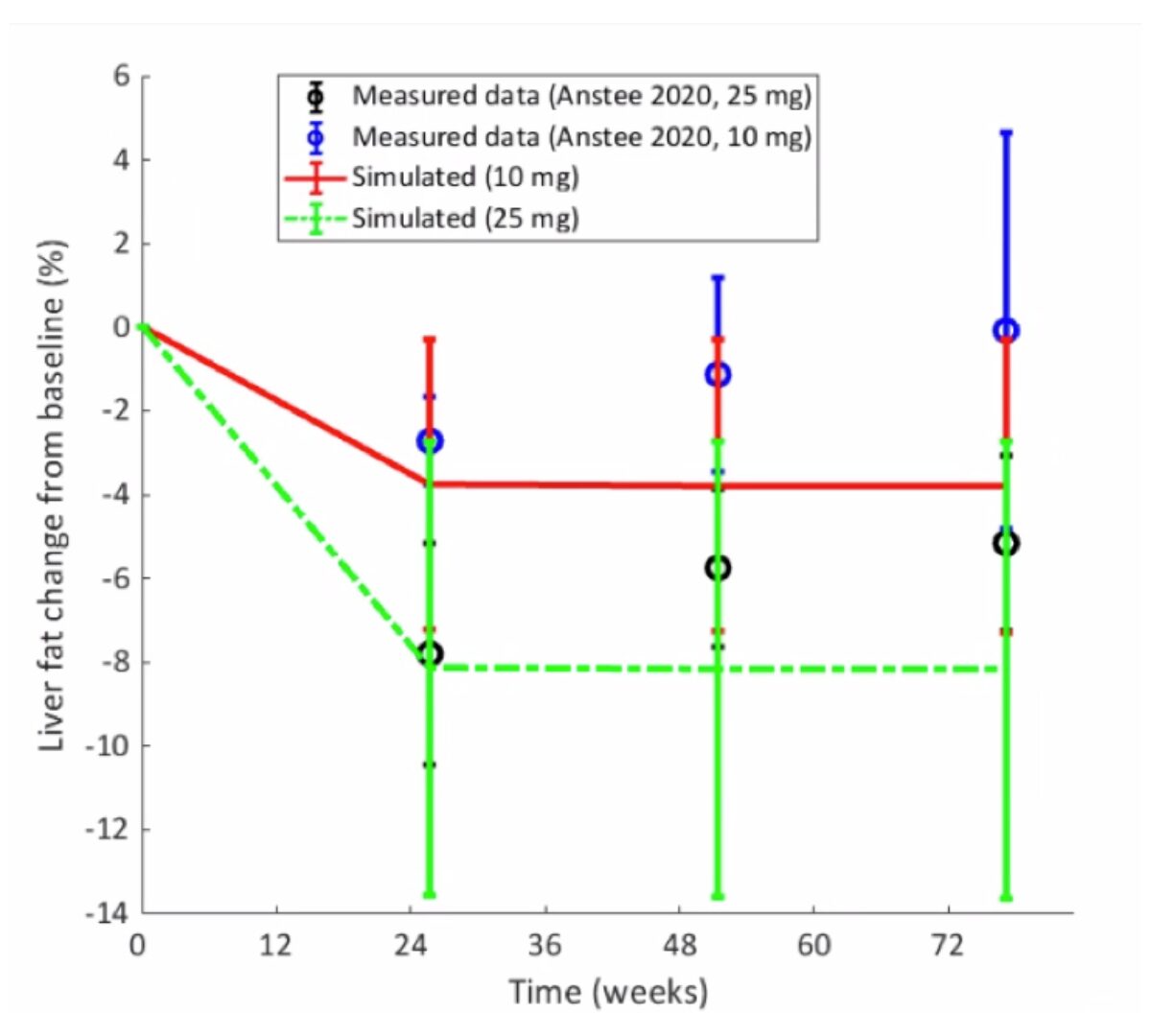
Predicting the Efficacy of Obeticholic Acid Treatment for Non-Alcoholic Steatohepatitis (NASH) Using NAFLDsym, a Quantitative Systems Pharmacology Model of Non-Alcoholic Fatty Liver Disease
Obeticholicacid (OCA), a bile acid analog and agonist of the farnesoidX receptor (FXR), is currently in clinical trials for the treatment of non-alcoholic steatohepatitis (NASH).

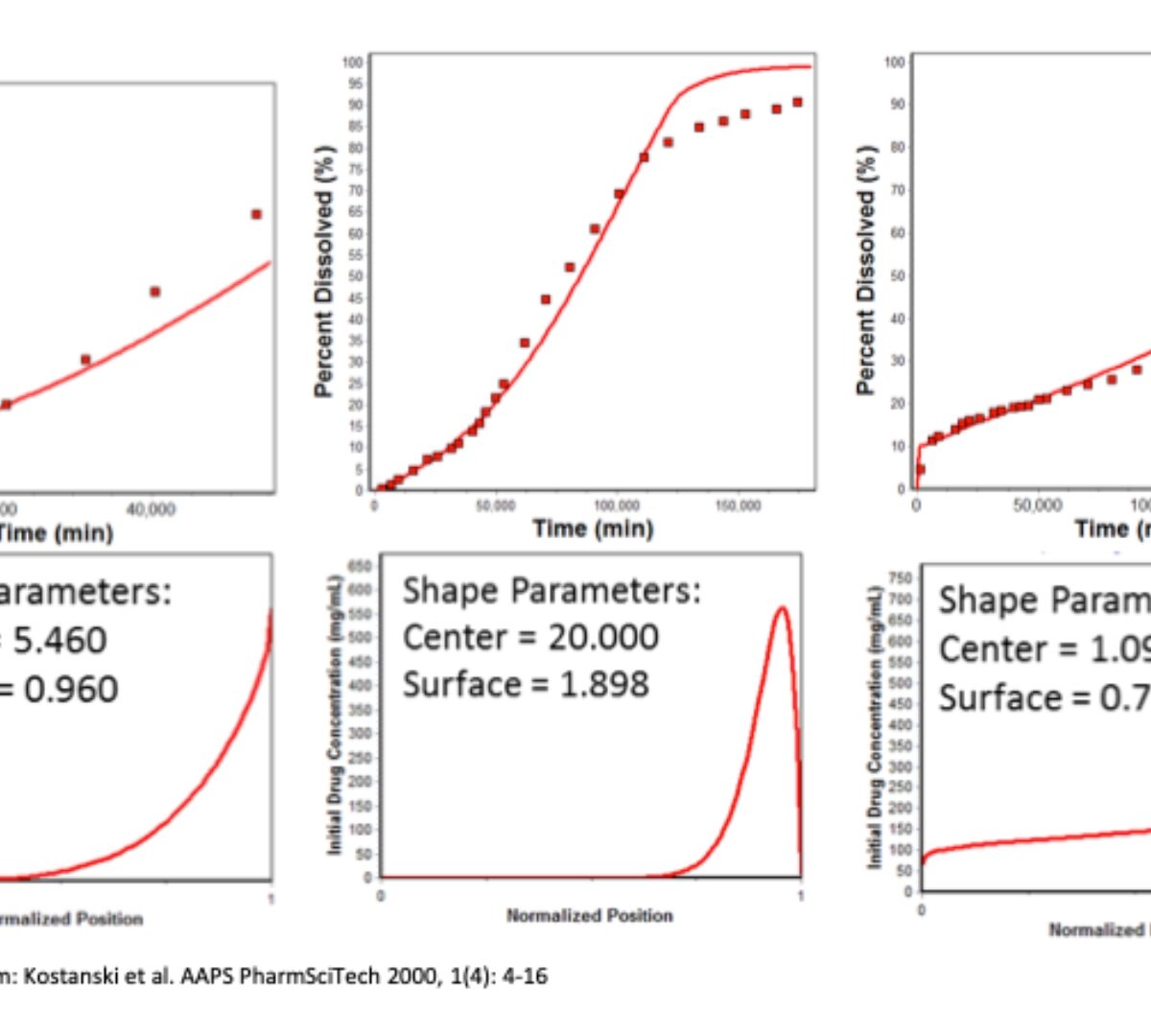
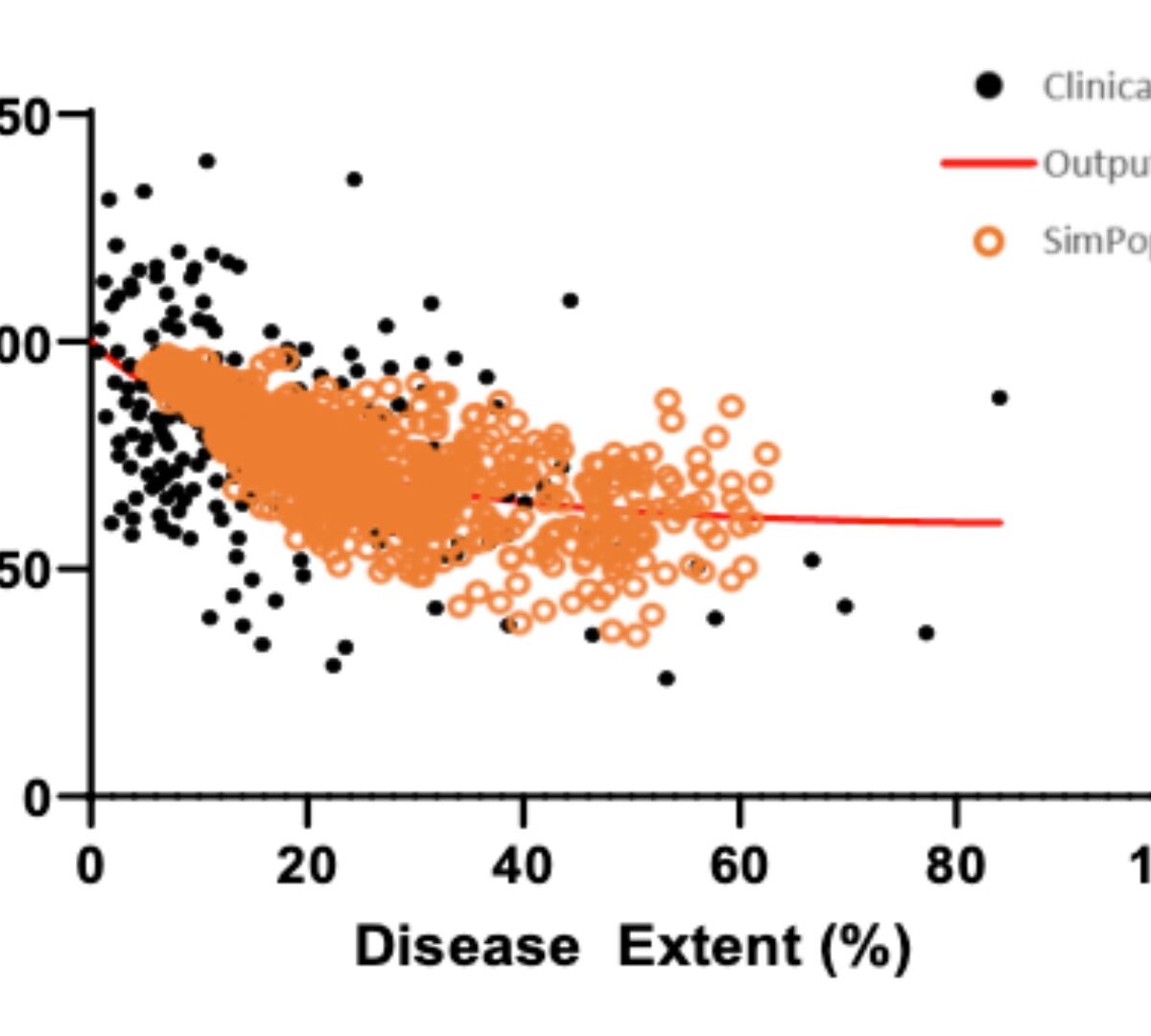
Establishment of preclinical mechanistic in vitro-in vivo correlations for long-acting injectable suspensions
Long acting injectable (LAI) formulations administered through subcutaneous (SC) or intramuscular (IM) routes provide sustained drug release over an extended period.
ILDsym®, a Quantitative Systems Pharmacology (QSP) Platform, Successfully Simulates the Pathophysiology of Systemic Sclerosis-Interstitial Lung Disease (SSc-ILD) and Inter-patient Variability
Systemic sclerosis (SSc) is a rare connective tissue and autoimmune disease associated with inflammation of the skin and internal organs.

Use of Quantitative Systems Toxicology (QST) to Identify Potential Intrinsic Mechanisms of Toxicity
BAY1128688, a selective inhibitor of aldo-keto reductase family 1 member C3 (AKR1C3), was in clinical trials as a potential therapy to provide pain relief for women with endometriosis.

October 2022 GastroPlus Newsletter
Breaking News! The new version of GP (version 9.8.3) is now available for download.

Simulations Plus Releases GastroPlus® Version 9.8.3
New update expands the library of virtual populations and enhances connections between software platforms

Utility of preclinical species for uncertainty assessment and correction of prediction of human volume of distribution using the Rodgers-Lukacova model
Prediction of rat, dog, monkey, and human volume of distribution (VDss) by Rodgers-Lukacova model was evaluated using a data set of more than 100 compounds.

The Requirements for Additional Strength Biowaivers for Immediate Release Solid Oral Dosage Forms in International Pharmaceutical Regulators Programme Participating Regulators and Organisations: Differences and Commonalities
In relation to the registration of generic products, waivers of in vivo bioequivalence studies (biowaivers) are considered in three main cases: certain dosage forms for which...

A Survey of the Criteria Used for the Selection of Alternative Comparator Products by Participating Regulators and Organizations of the International Pharmaceutical Regulators Programme
The safety and efficacy of a generic product are partly based on demonstrating bioequivalence to the innovator product; however, when the innovator product is no longer available as a comparator product...

In vitro antibacterial activity and in vivo pharmacokinetics of intravenously administered Amikacin-loaded Liposomes for the management of bacterial septicaemia
Systemic delivery of amikacin is a widely adopted treatment modality for severe infections like sepsis. However, the current course of treatment requires repeated bolus doses of amikacin, prolonged hospitalization, and continuous therapeutic monitoring to manage the severe adverse effects.

SeDeM tool-driven full factorial design for osmotic drug delivery of tramadol HCl: Formulation development, physicochemical evaluation, and in-silico PBPK modeling for predictive pharmacokinetic evaluation using GastroPlus™
An elementary osmotic pump (EOP) is the simplest form of osmotic drug delivery system that consists of the combination of active pharmaceutical ingredients and excipients...

Population pharmacokinetic modeling of daridorexant, a novel dual orexin receptor antagonist
The analysis aimed at identifying subject-specific characteristics (covariates) influ-encing exposure to daridorexant and quantification of covariate effects to determineclinical relevance.

Prediction of janagliflozin pharmacokinetics in type 2 diabetes mellitus patients with liver cirrhosis or renal impairment using a physiologically based pharmacokinetic model
Janagliflozin is a sodium-glucose cotransporter 2 (SGLT2) inhibitor for type 2 diabetes mellitus (T2DM).

Pimavanserin exposure–response analyses in patients with schizophrenia: results from the phase 2 ADVANCE study
Pimavanserin is a selective serotonin 5-HT2A receptor inverse agonist/antagonist being investigated in patients with negative symptoms of schizophrenia. This analysis aimed to characterize exposure-response relationships of...

Evaluating the bioequivalence of two pitavastatin calcium formulations based on IVIVC modeling and clinical study
In vitro-in vivo correlation (IVIVC) allows prediction of the in vivo performance of a pharmaceutical product based on its in vitro drug release profiles...