Although traditionally contraindicated, the coadministration of tamoxifen and estradiol may hold clinical relevance in specific contexts, particularly in breast cancer survivors with premature menopause...
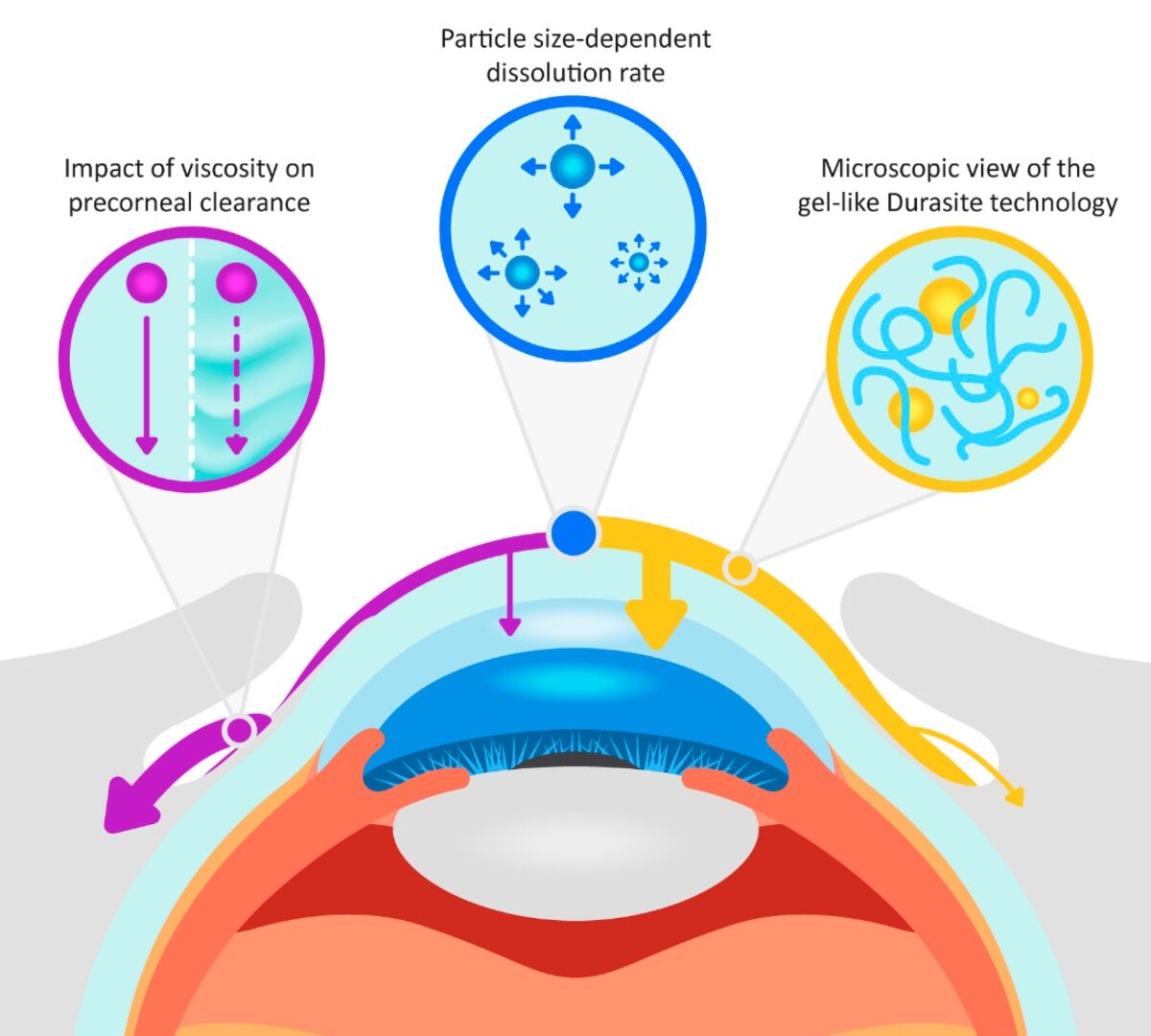
Get Under the Skin with GPX.2
If you’re working on the development of injectable therapies, then this webinar is for you

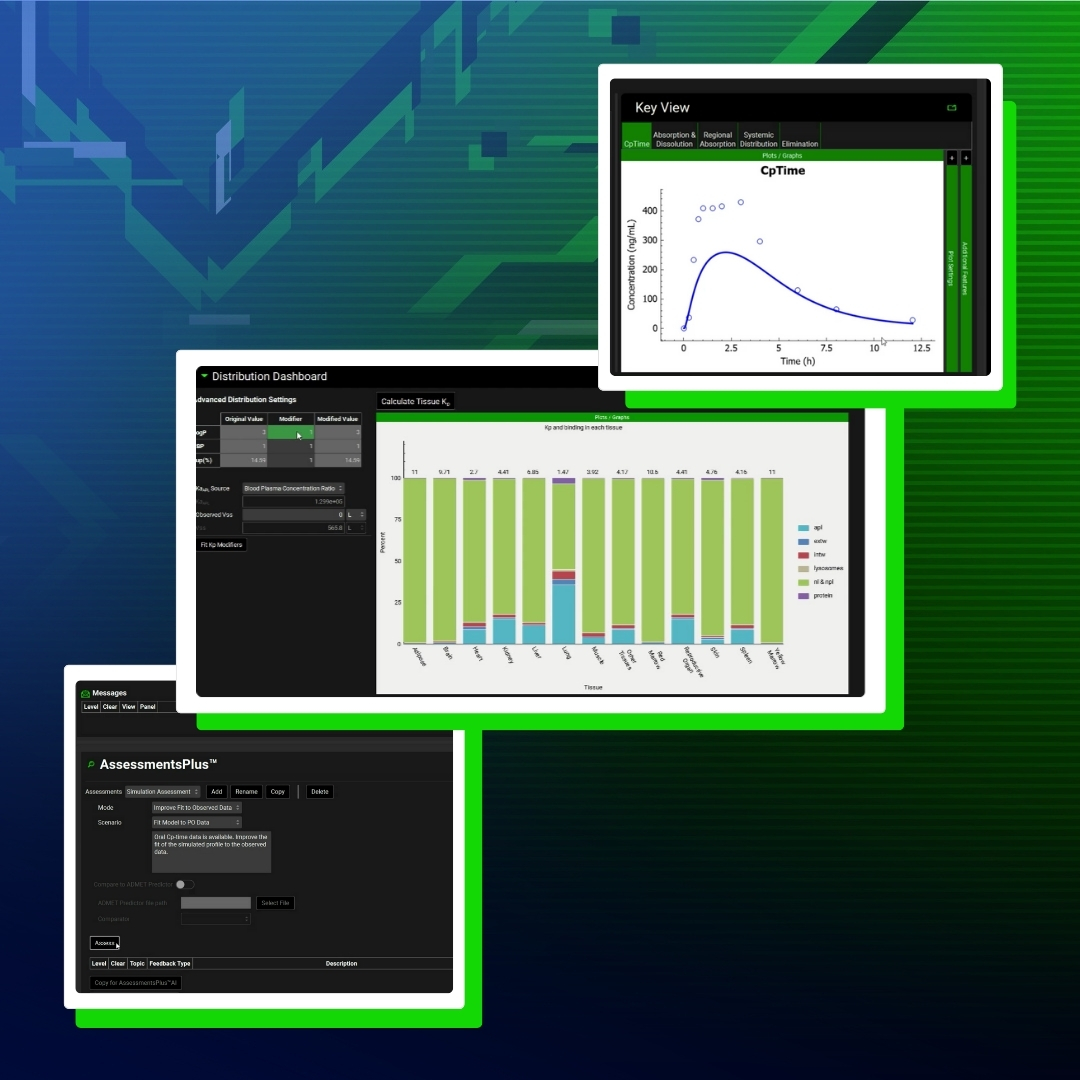
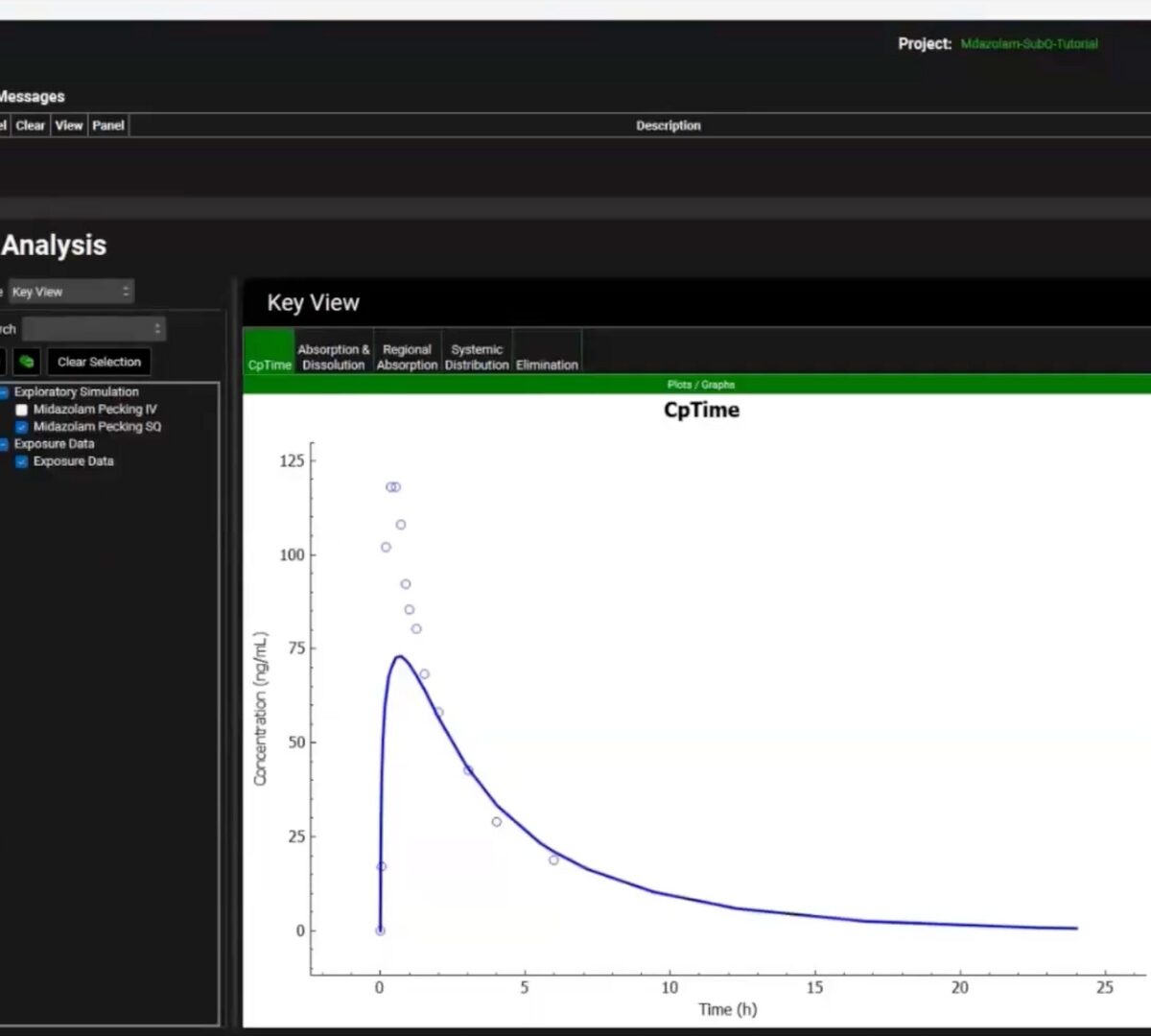
Introducing Orchestrator – Faster Workflows, Smarter Science
As scientists working in pre-clinical drug development, we are constantly balancing the need for rigorous, mechanistic modeling with the realities of fast-moving project timelines.
![Whole-Body Disposition and Metabolism of [14C]-2,4,4′-Trichlorobiphenyl (PCB28) Following Lung Administration in Rats](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
Whole-Body Disposition and Metabolism of [14C]-2,4,4′-Trichlorobiphenyl (PCB28) Following Lung Administration in Rats
Toxicities of lower-chlorinated biphenyls (LC-PCBs) have drawn increasing attention due to growing evidence of their presence in school indoor air, with 2,4,4′-trichlorobiphenyl (PCB28) being a prevalent congener.

Novel Workflow for Non-Animal PBK Modelling of UV Filters: Oxybenzone as a Case Study
Physiologically based kinetics (PBK) modelling provides (internal) exposure concentrations. We used a PBK model parameterized exclusively with in silico and in vitro data in a bottom-up approach to predict the pharmacokinetics of oxybenzone, a UV filter, present in two formulations (for which dose-normalized Cmax and AUC from clinical studies were different).

Simulations Plus Announces Preliminary Fiscal Year 2025 Results and Fiscal Year 2026 Guidance
Provides preliminary fiscal 2026 revenue guidance of $79 to $82 million and adjusted diluted EPS guidance of $1.03 to $1.10
Fourth quarter and fiscal year 2025 results to be reported December 1, 2025

Emerging Perspectives on Leveraging Physiologically Based Biopharmaceutics Modeling (PBBM) for BCS Class III Biowaivers: a Webinar Summary
The regulatory framework for Biopharmaceutics Classification System (BCS) class III drug products provides a pathway for streamlined biowaivers in drug development, eliminating the need for expensive...
QSAR-based Physiologically Based Pharmacokinetic (PBPK) Modeling for 34 Fentanyl Analogs: Model Validation, Human Pharmacokinetic Prediction and Abuse Risk Insights
Fentanyl analogs, as emerging new psychoactive substances (NPS), pose a global public health threat due to widespread abuse, high toxicity, and frequent overdose fatalities.
AI-Driven Knowledge Management in PBPK Modeling: Challenges & Opportunities
High-quality data is the foundation of every major AI advance
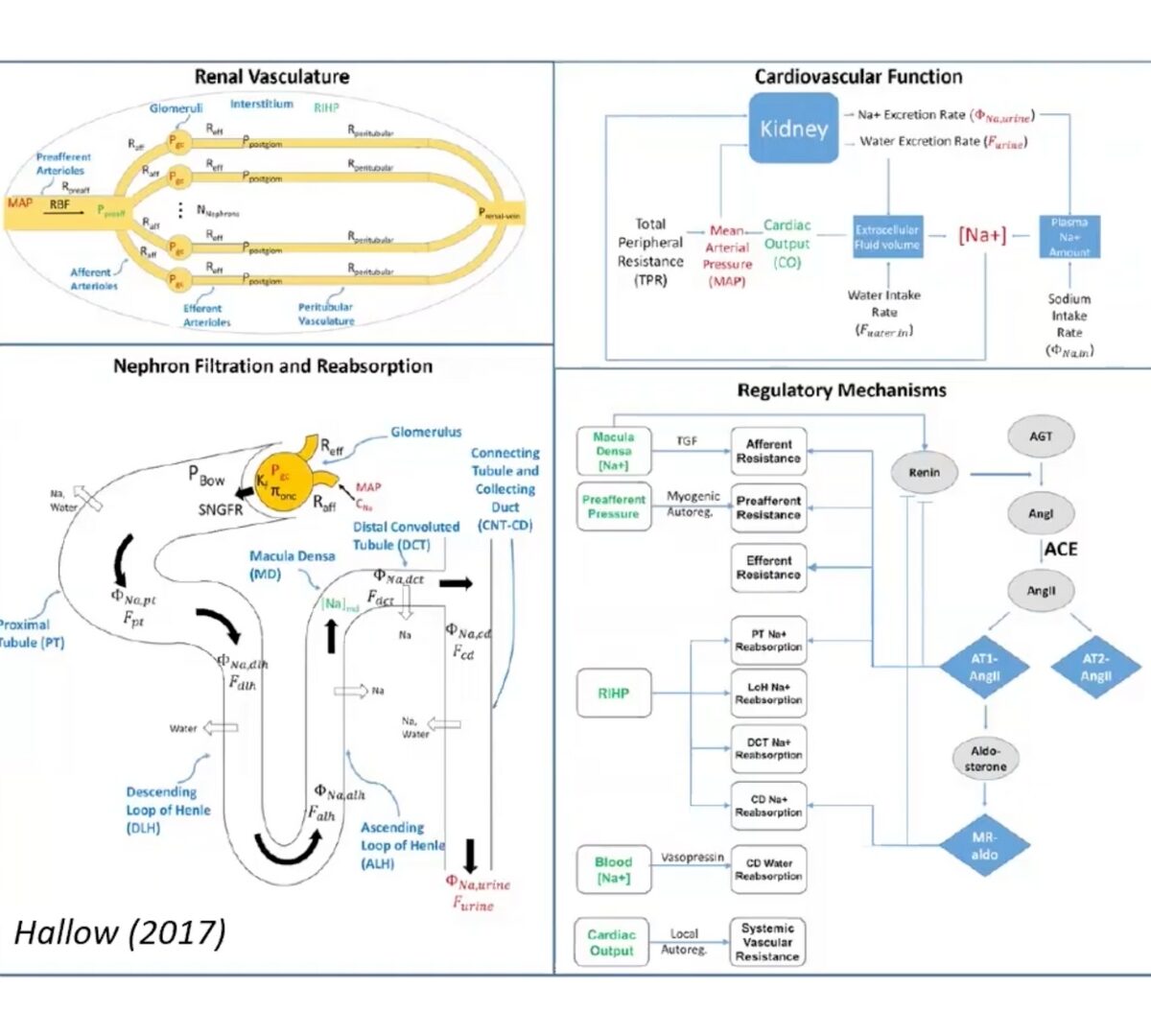
Advancing Kidney Safety: What’s New in RENAsym® 2A
Drug-induced kidney injury remains a significant concern in pharmaceutical development, with traditional methods often falling short in predicting renal toxicity across diverse compounds and patient populations.

Development of Co-Amorphous Systems for Inhalation Therapy—Part 2: In Silico Guided Co-Amorphous Rifampicin–Moxifloxacin and –Ethambutol Formulations
Tuberculosis (TB) remains a global health challenge due to long treatment durations, poor adherence, and growing drug resistance. Inhalable co-amorphous systems (COAMS) offer a promising strategy for targeted pulmonary delivery...
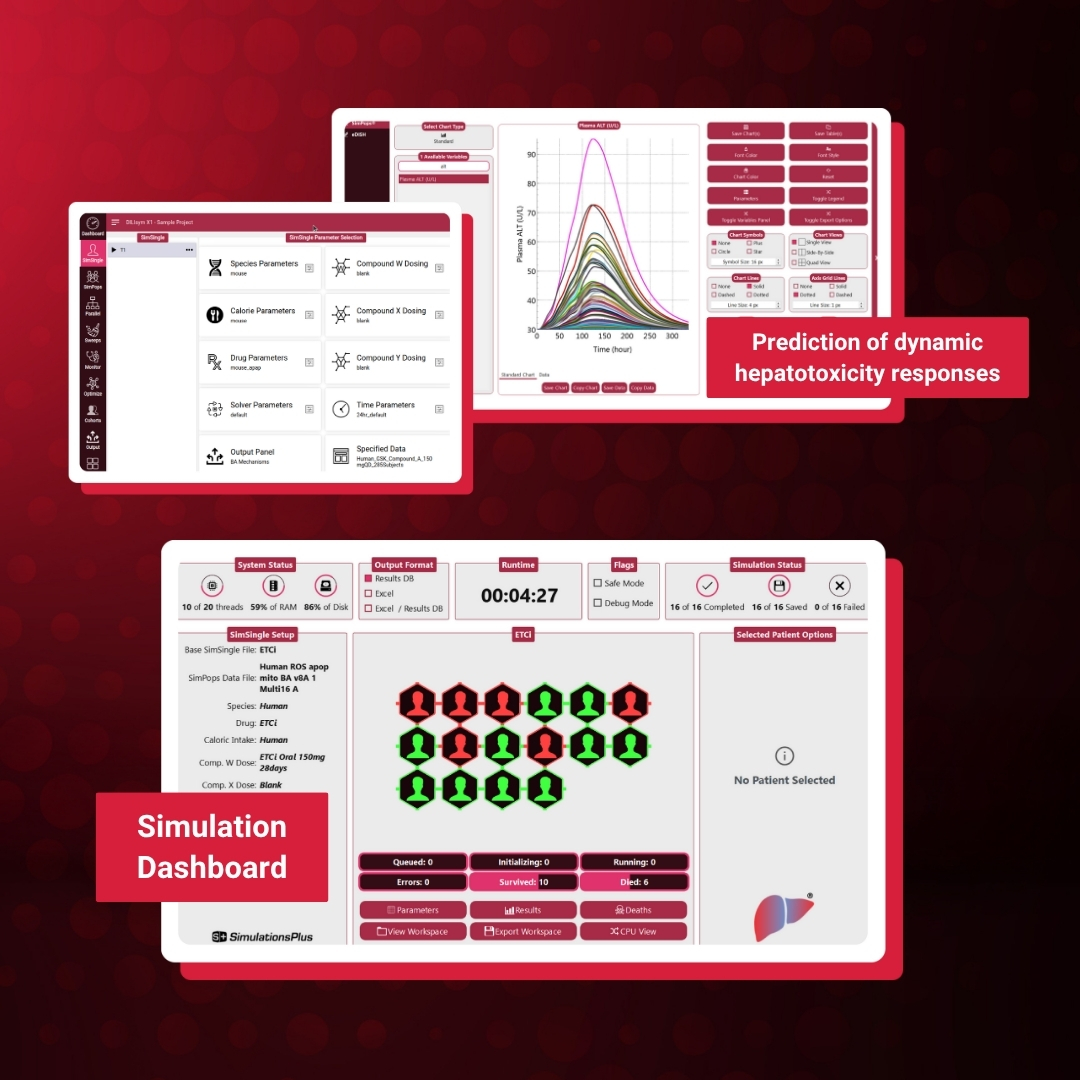
DILIsym® 11 Product Brochure
Quantitative Systems Toxicology (QST) Software for Predicting and Explaining Drug-Induced Liver Injury (DILI)
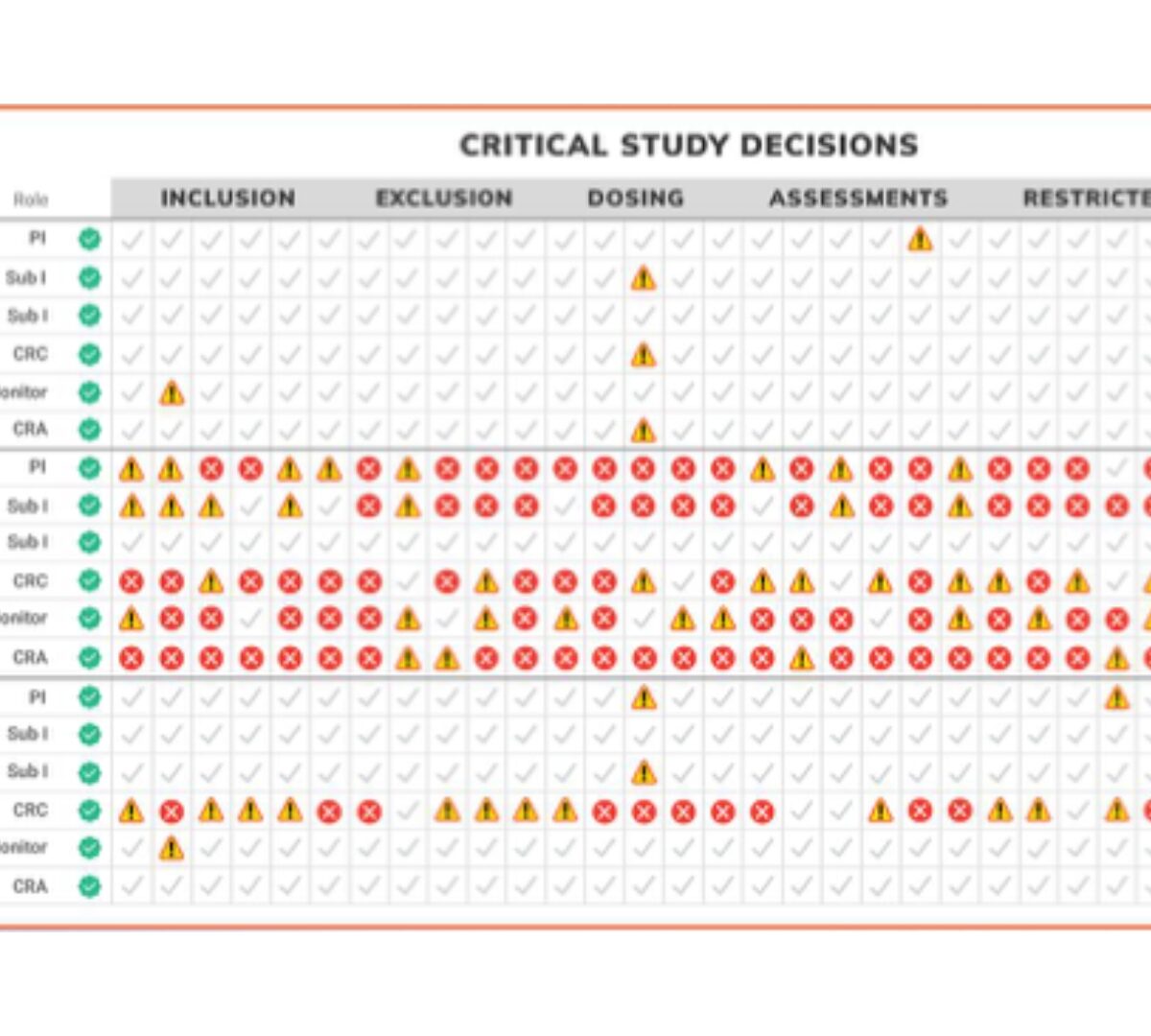
The Impact of Simulation-Based Learning on Study Acceleration: Spoken from the Sponsor who Converted
Enhancing Training to be a personalized, representative and interactive strategic asset

Expanding ADMET Predictor®’s Chemical Space: Enhanced bRo5 and Chameleon Molecule Predictions for HTPK
ADMET Predictor has been enhanced to accurately predict properties of beyond Rule-of-Five (bRo5) molecules, including macrocycles and PROTACs.

Formulation and Evaluation of Poly(Jasmine Lactone) Based Micelles for Improving the Oral Permeability of Acyclovir
Acyclovir (ACV), an antiviral drug, belongs to the BCS class III drug with intermediate solubility and low permeability.

Simulation-Guided Dissolution Testing: Coupling DDDPlus™ and GastroPlus® to Predict Aripiprazole Oral Bioperformance
Orally administered weakly basic compounds like aripiprazole (ARI) can precipitate in the small intestine due to limited solubility at intestinal pH.

Sensitivity Analysis of the Inputs for Bioactivity-Exposure Ratio Calculations in a NAM-based Systemic Safety Toolbox
To support regulatory decision-making without animal testing, Next-Generation Risk Assessment (NGRA) frameworks leverage New Approach Methodologies (NAM).

3 Mistakes We’re Still Making with Study Training (and How to Fix Them)
Training is one of the most critical parts of study start-up and yet, we often treat it like a box to check rather than the foundation of trial quality.

Applications of PBPK Models to Predict Tissue Residues and Extralabel Withdrawal Times of Drugs in Food Animals: Perspectives from the Food Animal Residue Avoidance Databank (FARAD) Program
Physiologically based pharmacokinetic (PBPK) models are commonly used in human drug discovery and development and human health risk assessment of environmental chemicals.