Explore modeling and simulation summer school opportunities inside

The Combination of a Human Biomimetic Liver Microphysiology System with BIOLOGXsym, a Quantitative Systems Toxicology (QST) Modeling Platform for Macromolecules, Provides Mechanistic Understanding of Tocilizumab- and GGF2-Induced Liver Injury
Biologics address a range of unmet clinical needs, but the occurrence of biologics-induced
liver injury remains a major challenge. Development of cimaglermin alfa (GGF2) was terminated...
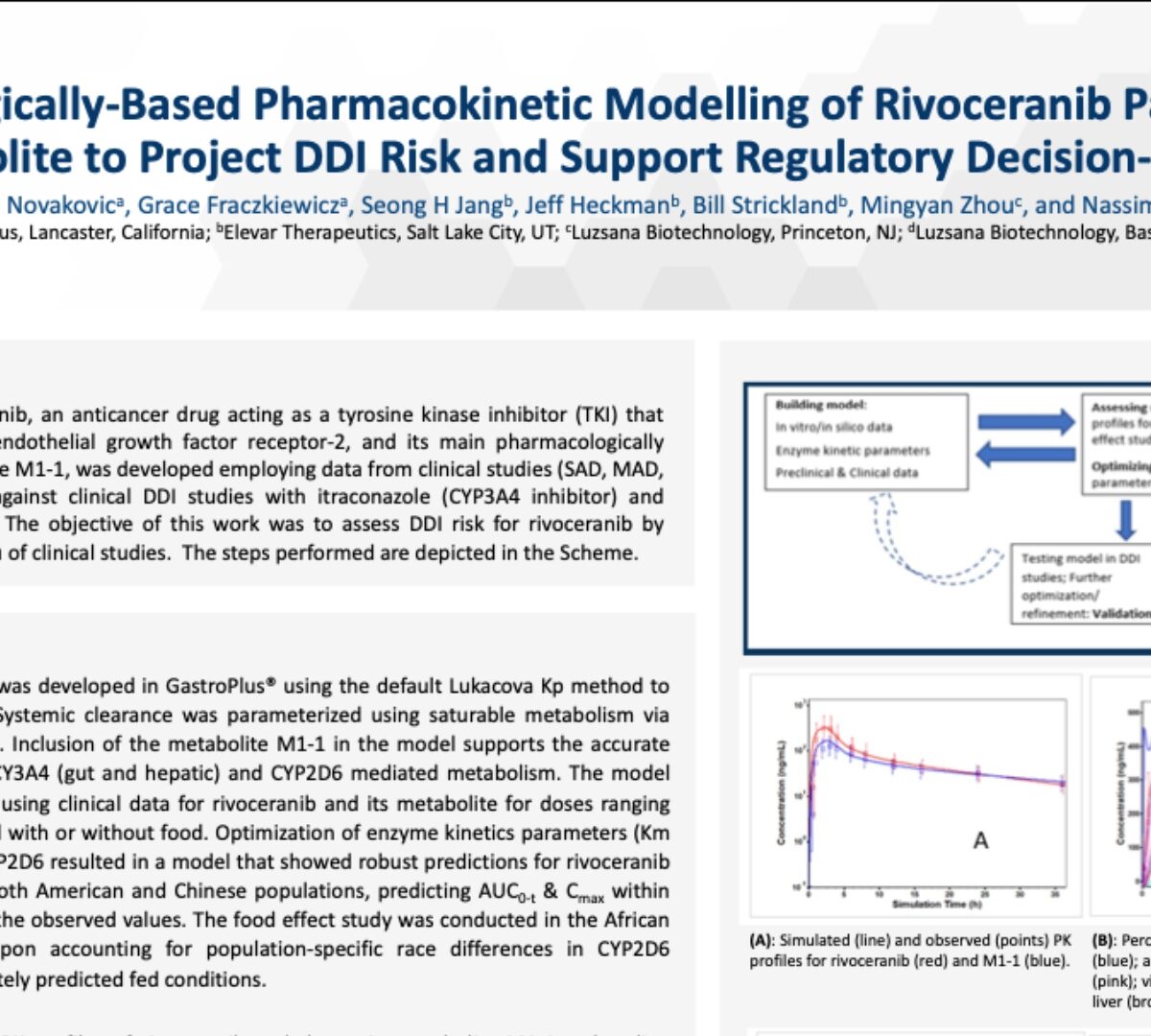
Physiologically-Based Pharmacokinetic Modelling of Rivoceranib Parent and Metabolite to Project DDI Risk and Support Regulatory Decision-Making
The PBPK model for rivoceranib, an anticancer drug acting as a tyrosine kinase inhibitor (TKI) that selectively targets vascular...

Development of a 2D-QSAR Model for Tissue-to-Plasma Partition Coefficient Value with High Accuracy Using Machine Learning Method, Minimum Required Experimental Values, and Physicochemical Descriptors
The demand for physiologically based pharmacokinetic (PBPK) model is increasing currently.
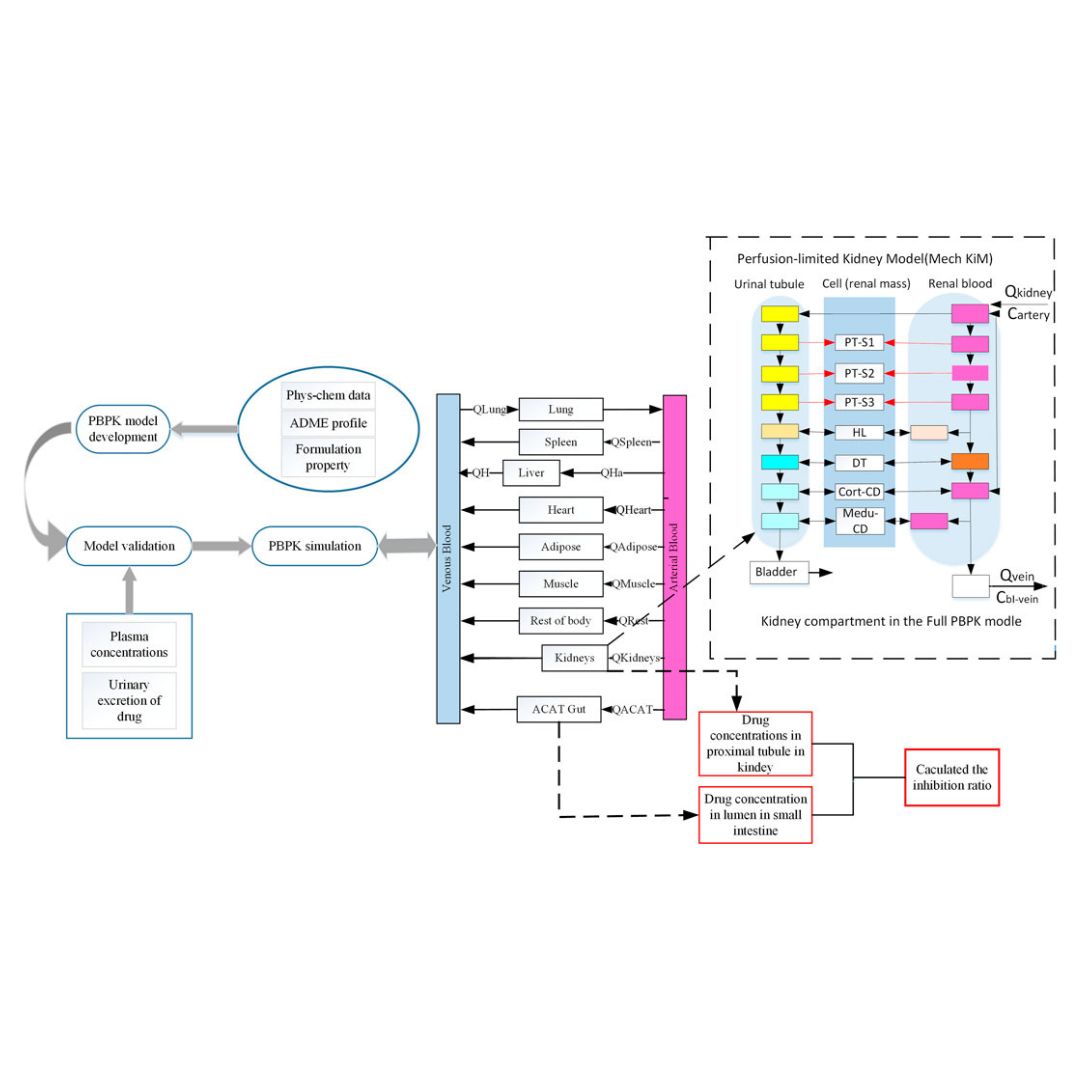
Mechanistic evaluation of the inhibitory effect of four SGLT-2 inhibitors on SGLT 1 and SGLT 2 using physiologically based pharmacokinetic (PBPK) modeling approaches
Sodium-glucose co-transporter type 2 (SGLT 2, gliflozins) inhibitors are potent orally active drugs approved for managing type 2 diabetes.
Applying Mechanistic PBPK Modeling and Simulations to Support Regulatory Interactions
Physiologically-Based Pharmacokinetic (PBPK) Modeling is a Tool/Component of Model-Informed Drug Development (MIDD)
Using AI-driven Drug Design to Shorten Your Drug Development Process
In this webinar, Dr. Jeremy Jones, Principal Scientist, will discuss how artificial intelligence (AI) can be used in the drug discovery and development process to identify viable candidate molecules and shorten time to market.
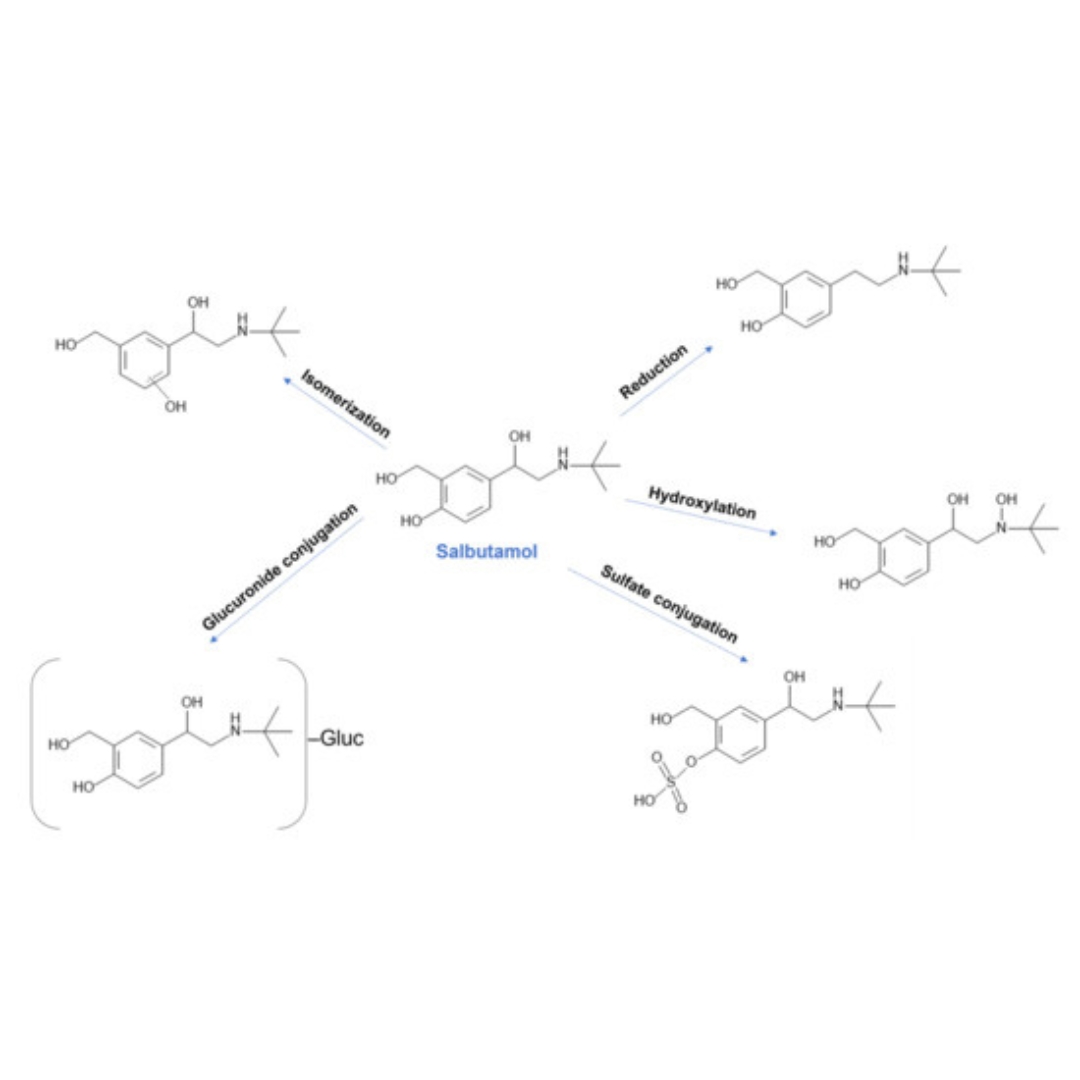
Prediction of CYP-Mediated Drug Interaction Using Physiologically Based Pharmacokinetic Modeling: A Case Study of Salbutamol and Fluvoxamine
Drug–drug interactions (DDIs) represent a significant concern in healthcare, particularly for patients undergoing polytherapy.
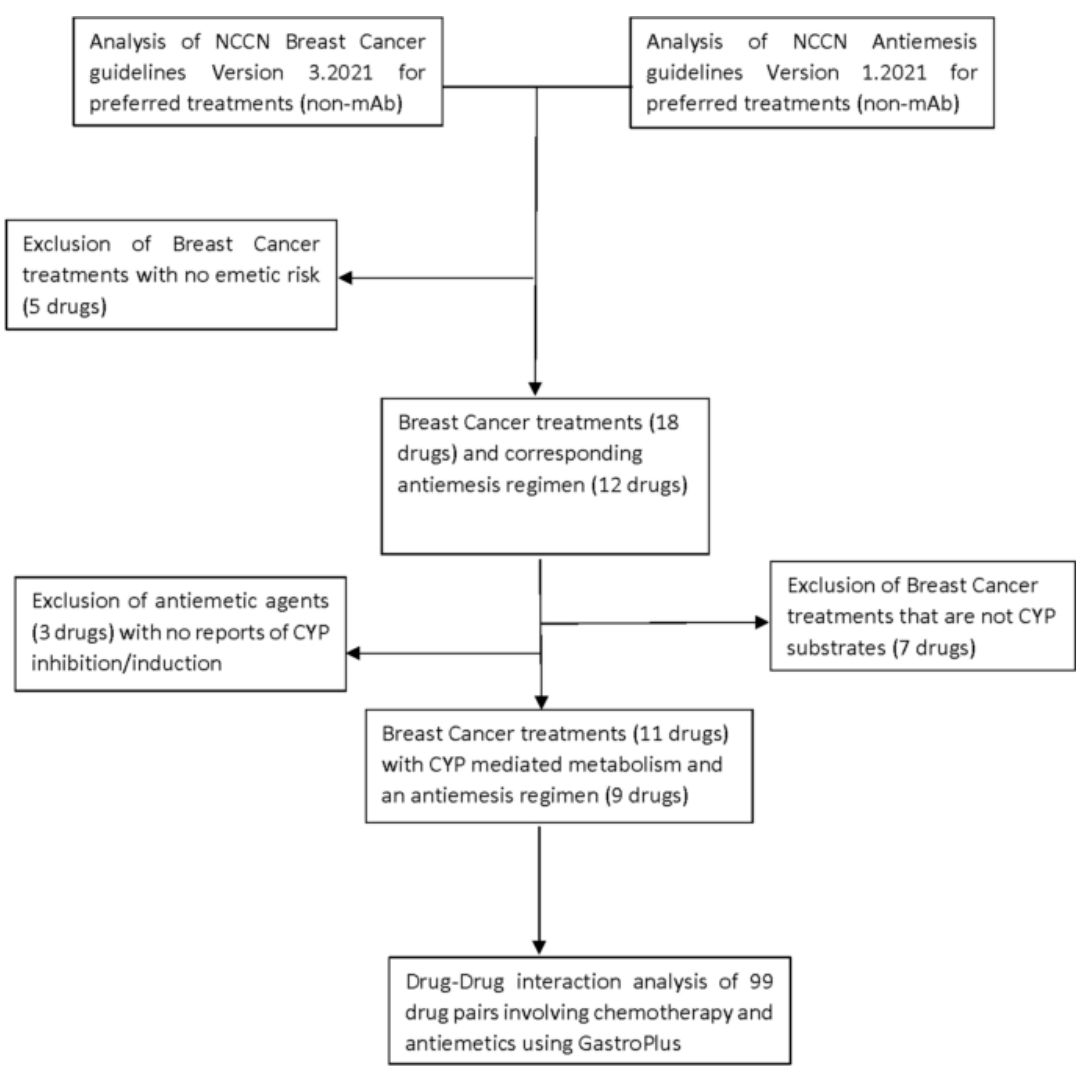
Simulation of drug-drug interactions between breast cancer chemotherapeutic agents and antiemetic drugs
Chemotherapy-induced nausea and vomiting are commonly experienced side effects in breast cancer (BCa) patients.

QSP Software and Service Flyer
Optimize your clinical trial design and decision making with Quantitative Systems Pharmacology (QSP)
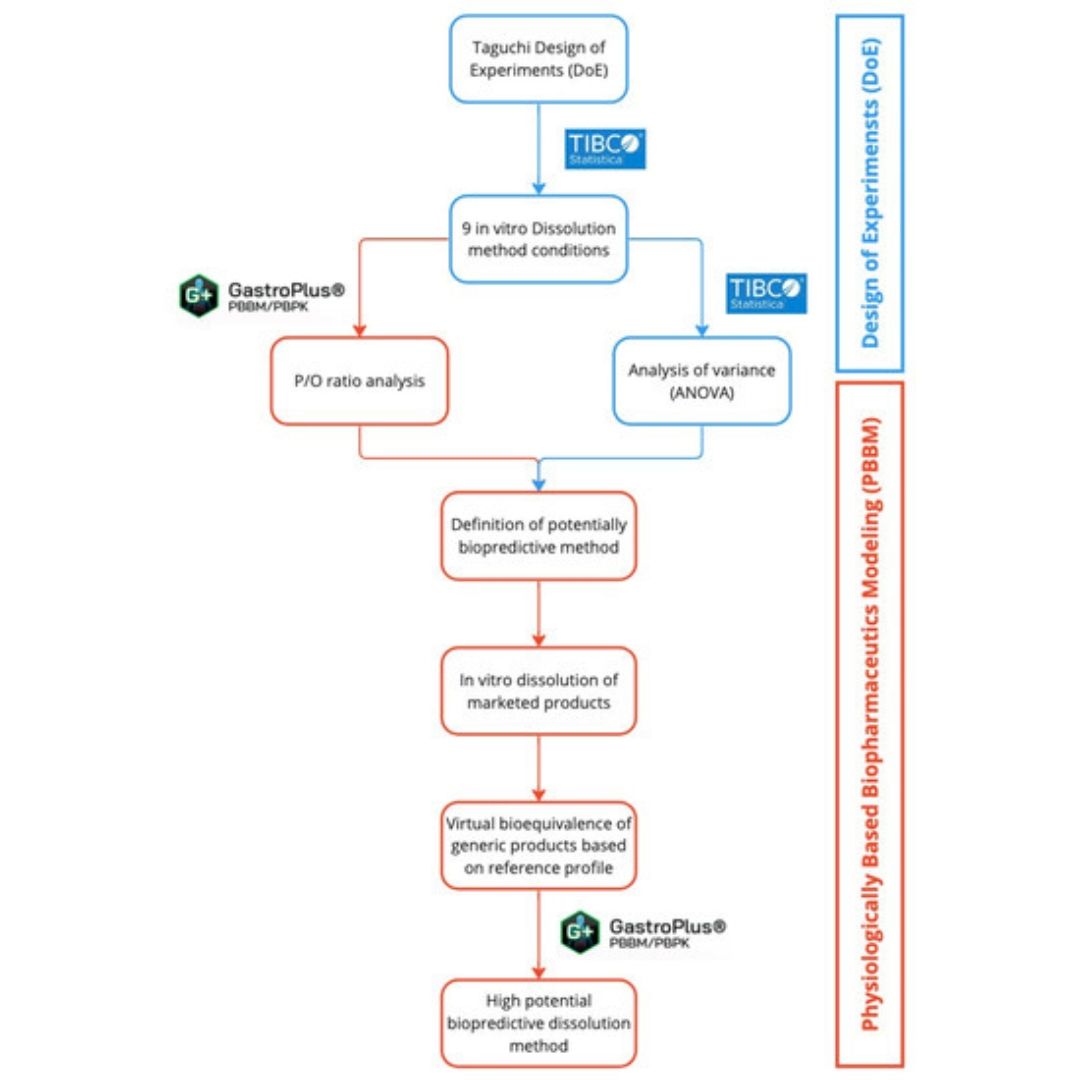
Development of Biopredictive Dissolution Method for Extended-Release Desvenlafaxine Tablets
This study aimed to develop a biopredictive dissolution method for desvenlafaxine ER tablets using design of experiments (DoE)...
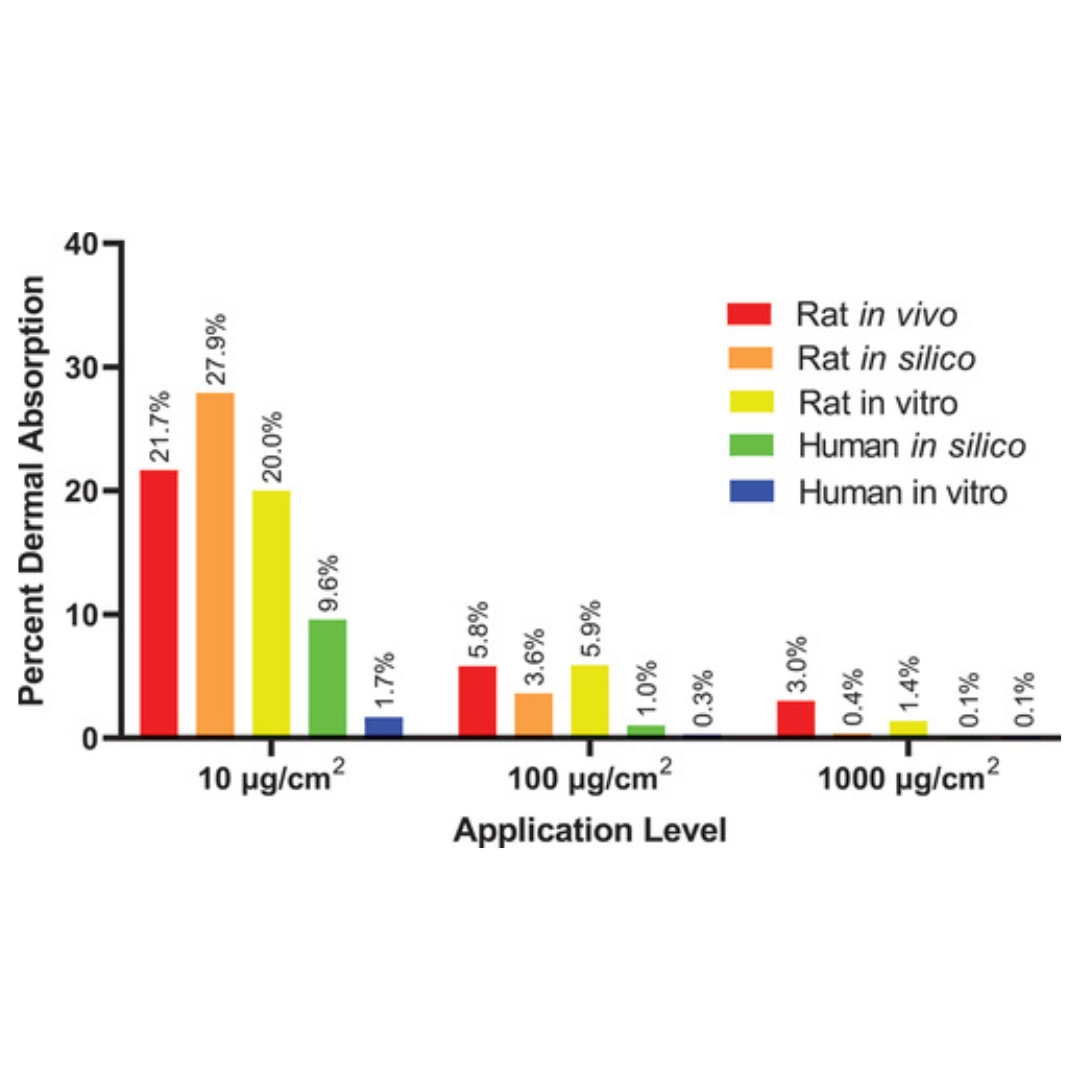
Estimated Dermal Penetration of Tetrachlorvinphos (TCVP) in Humans Based on In Silico Modeling and In Vitro and In Vivo Data
Tetrachlorvinphos (TCVP) is the pesticidal active ingredient in some collars for dogs and cats.
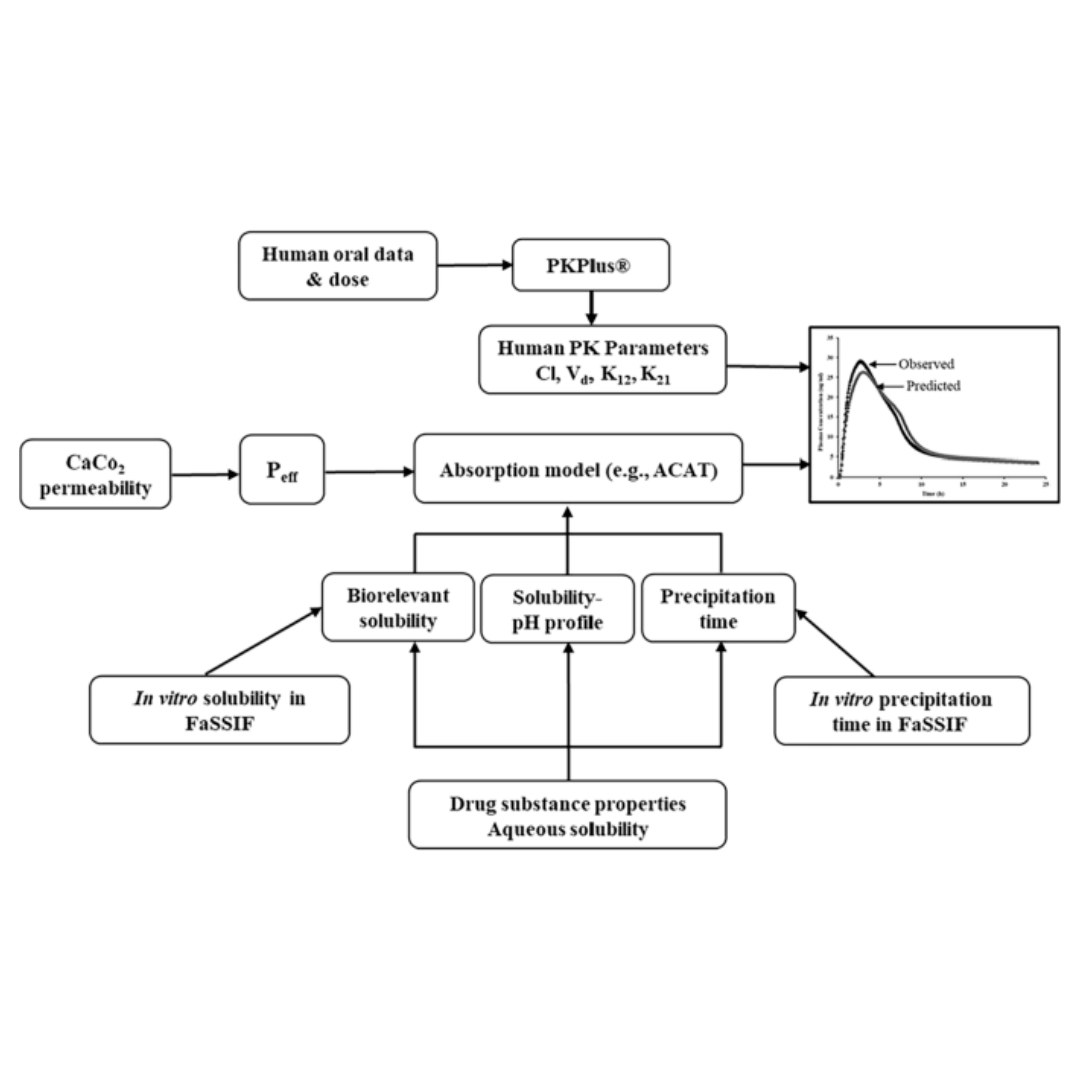
A Combined In-Vitro and GastroPlus® Modeling to Study the Effect of Intestinal Precipitation on Cinnarizine Plasma Profile in a Fasted State
Poorly water-soluble weak base molecules such as cinnarizine often exhibit pH-dependent solubility within the gastrointestinal tract.

Simulations Plus Launches New Integrated Pulmonary Software and Services Package to Streamline Drug Development and Improve Patient Outcomes
Simulations Plus, Inc. (Nasdaq: SLP), a leading provider of modeling and simulation software and services for pharmaceutical safety and efficacy, today announced the release of a new integrated pulmonary software and services package.

May 2023 GastroPlus Newsletter
Happy Spring… even though this year I have experienced the most severe case of “hay fever” ever.

DDDPlus™ Product Brochure
Simulations software for the in vitro dissolution experiment of pharmaceutical dosage forms
Subtractive sequence-mediated therapeutic targets from the conserved gene clusters of Campylobacter hyointestinalis and computational inhibition assessment
Campylobacter hyointestinalis is a causative agent of enteritis, proctitis, human gastroenteritis, and diarrhea.
Pan-genome mediated therapeutic target mining in Kingella kingae and inhibition assessment using traditional Chinese medicinal compounds: an informatics approach
Kingella kingae causes bacteremia, endocarditis, osteomyelitis, septic arthritis, meningitis, spondylodiscitis, and lower respiratory tract infections in pediatric patients
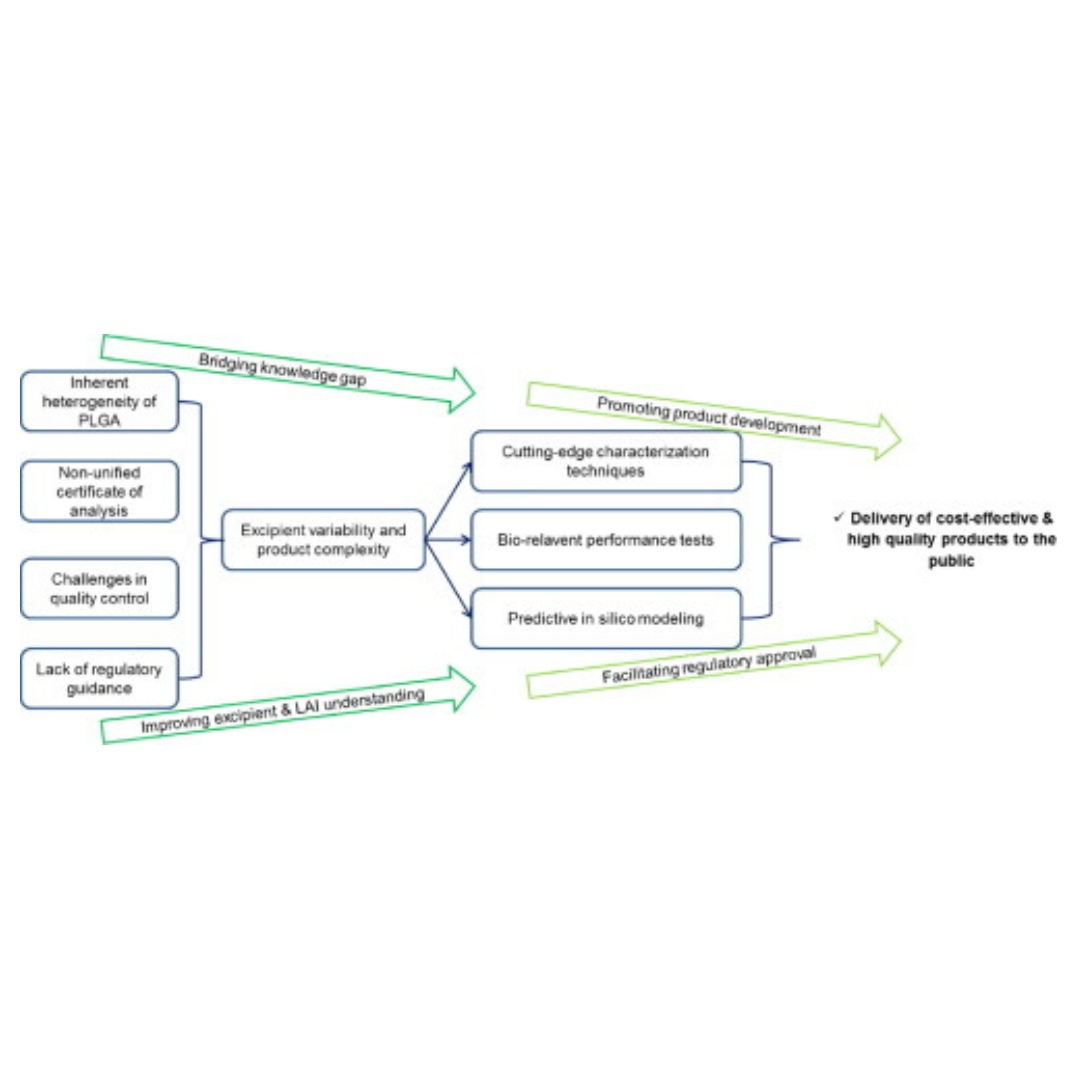
Long-acting PLGA microspheres: Advances in excipient and product analysis toward improved product understanding
Poly(lactic-co-glycolic acid) (PLGA) microspheres are a sustained-release drug delivery system with several

