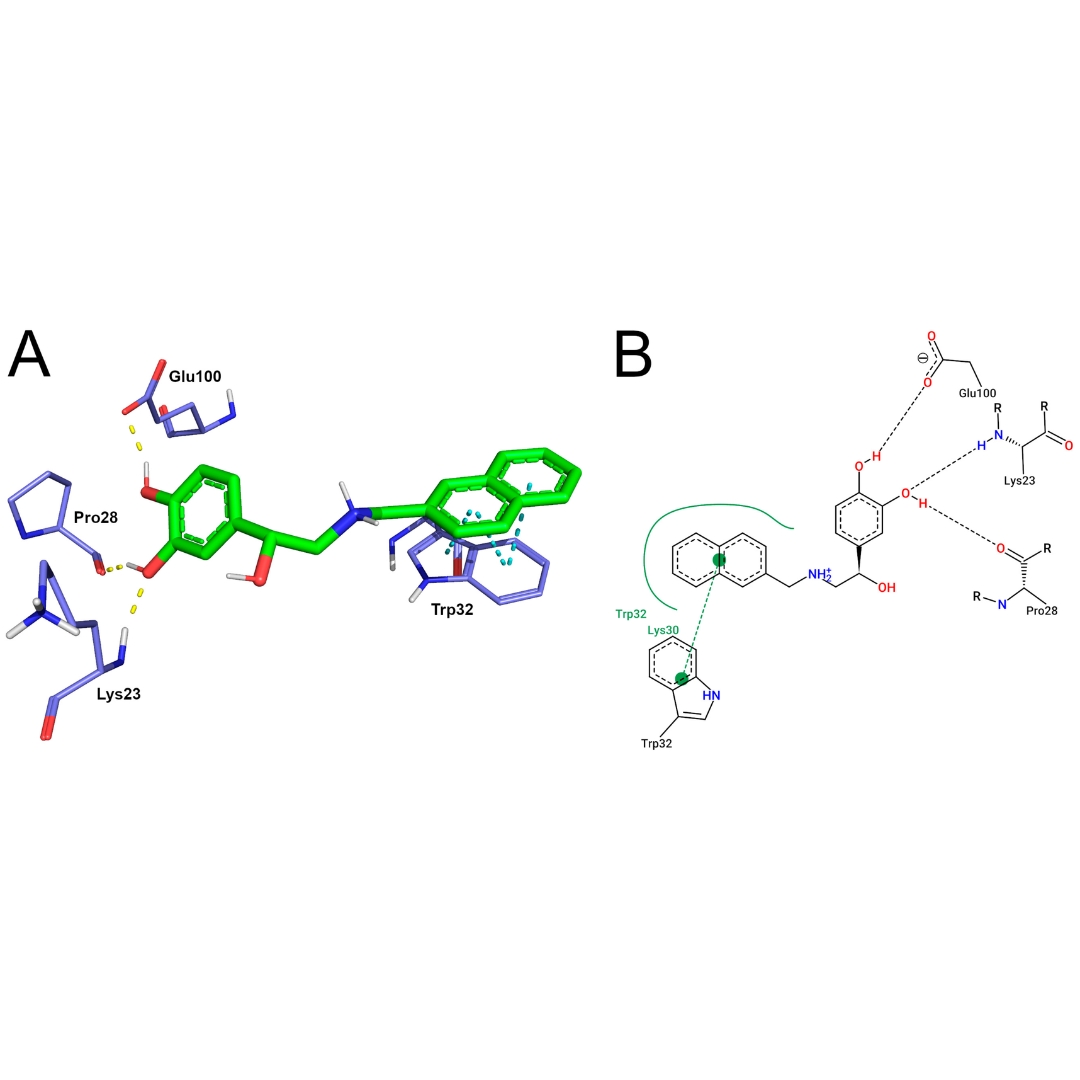
Amyotrophic lateral sclerosis (ALS) is the most prevalent motor neuron disorder in adults, which is associated with a highly disabling condition
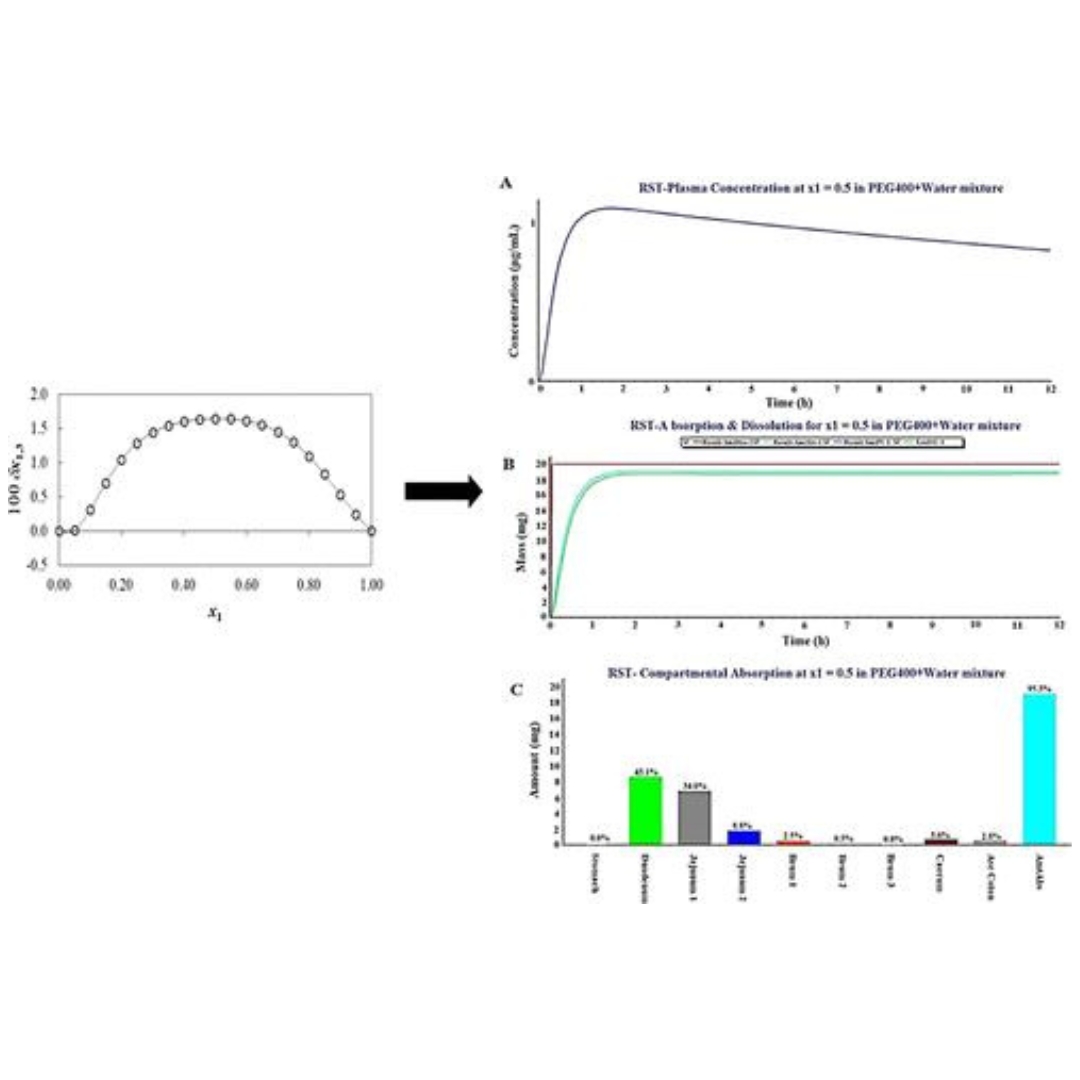
Preferential Solvation Study of Rosuvastatin in the {PEG400 (1) + Water (2)} Cosolvent Mixture and GastroPlus Software-Based In Vivo Predictions
Rosuvastatin (RST) is a poorly water-soluble drug responsible for limited in vivo dissolution and subsequently low oral systemic absorption (poor bioavailability).

Simulations Plus Enters New Strategic Collaboration to Discover Anticancer Therapies Through Its AI-Driven Drug Design Technology
Drug discovery services partnership with Sino-American Cancer Foundation focuses on the development of actionable hits against the MTHFD2 target
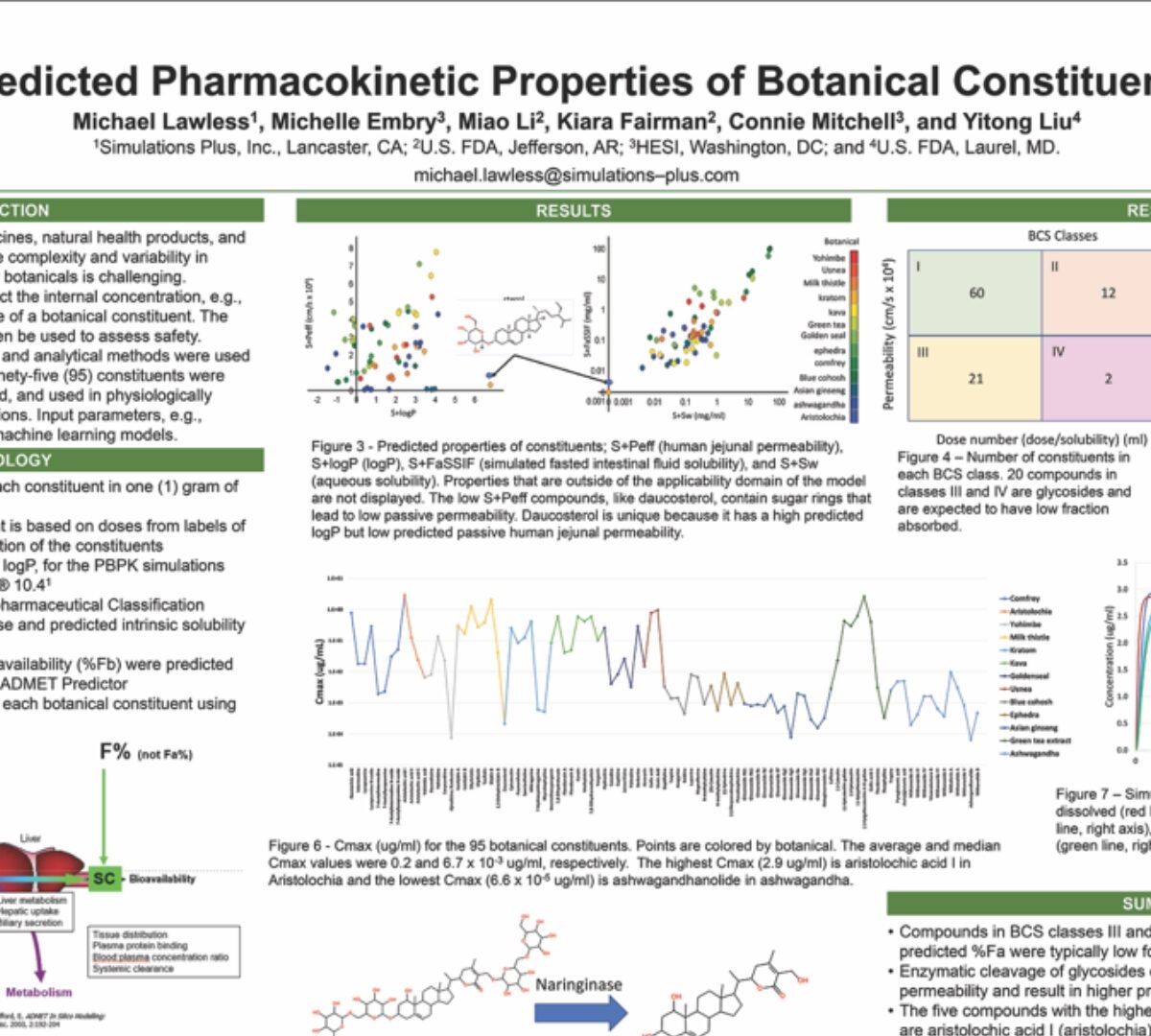
Predicted Pharmacokinetic Properties of Botanical Constituents
Botanicals are used as traditional medicines, natural health products, and dietary supplements globally.
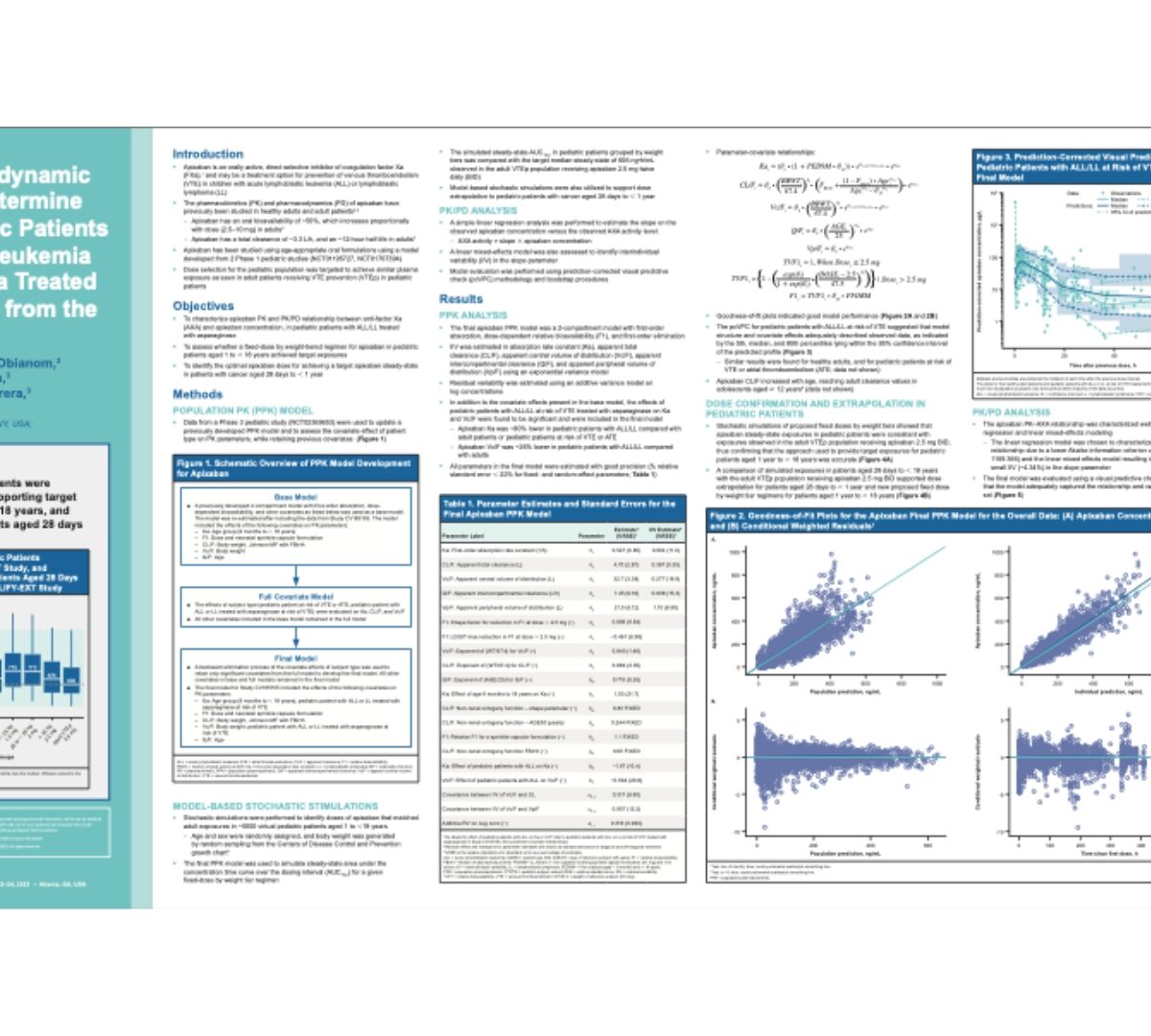
Pharmacokinetic/pharmacodynamic analysis of apixaban to determine dosing regimens for pediatric patients with acute lymphoblastic leukemia or lymphoblastic lymphoma treated with asparaginase: analysis from the PREVAPIX study
Apixaban is an orally active, direct selective inhibitor of coagulation factor Xa (FXa),1 and may be a treatment option for prevention of venous thromboembolism (VTE) in children with...
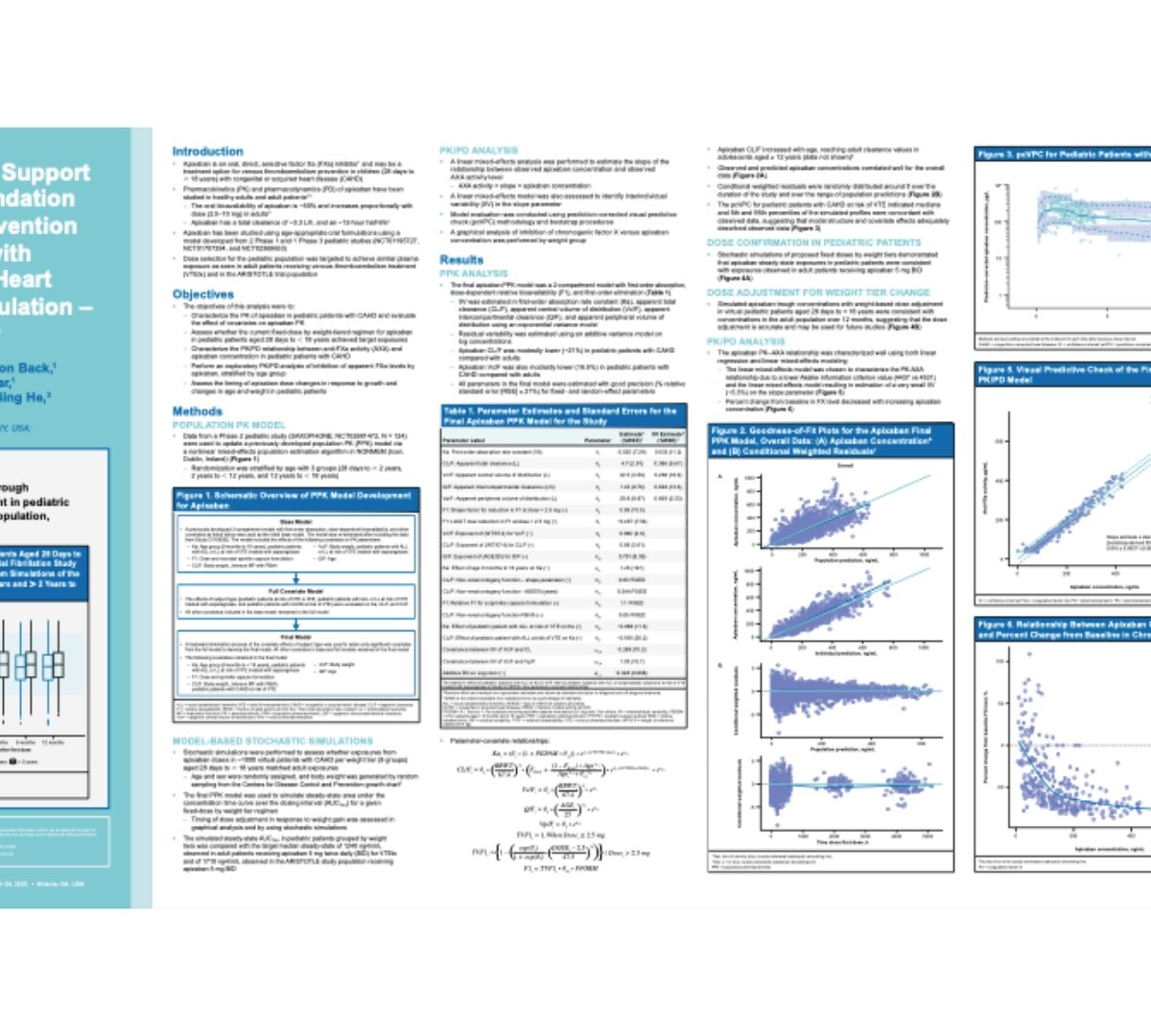
Modeling and simulation to support apixaban dose recommendation for thromboembolism prevention in pediatric subjects with congenital or acquired heart disease requiring anticoagulation–saxophone study
Apixaban is an oral, direct, selective factor Xa (FXa) inhibitor1 and may be a treatment option for venous thromboembolism prevention in...
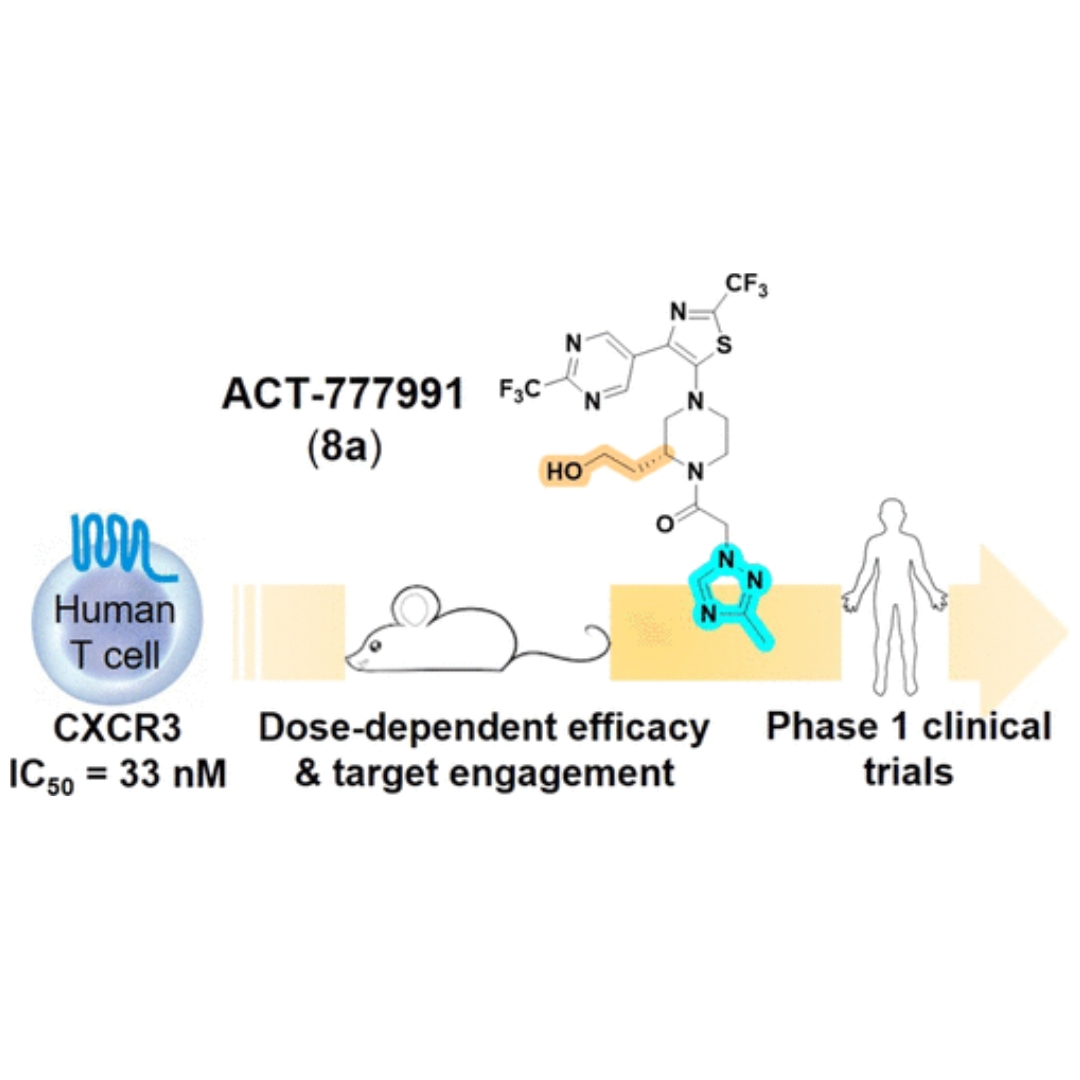
Discovery of Clinical Candidate ACT-777991, a Potent CXCR3 Antagonist for Antigen-Driven and Inflammatory Pathologies
The CXCR3 chemokine receptor is a G protein-coupled receptor mainly expressed on immune cells from the lymphoid lineage, including activated T cells.
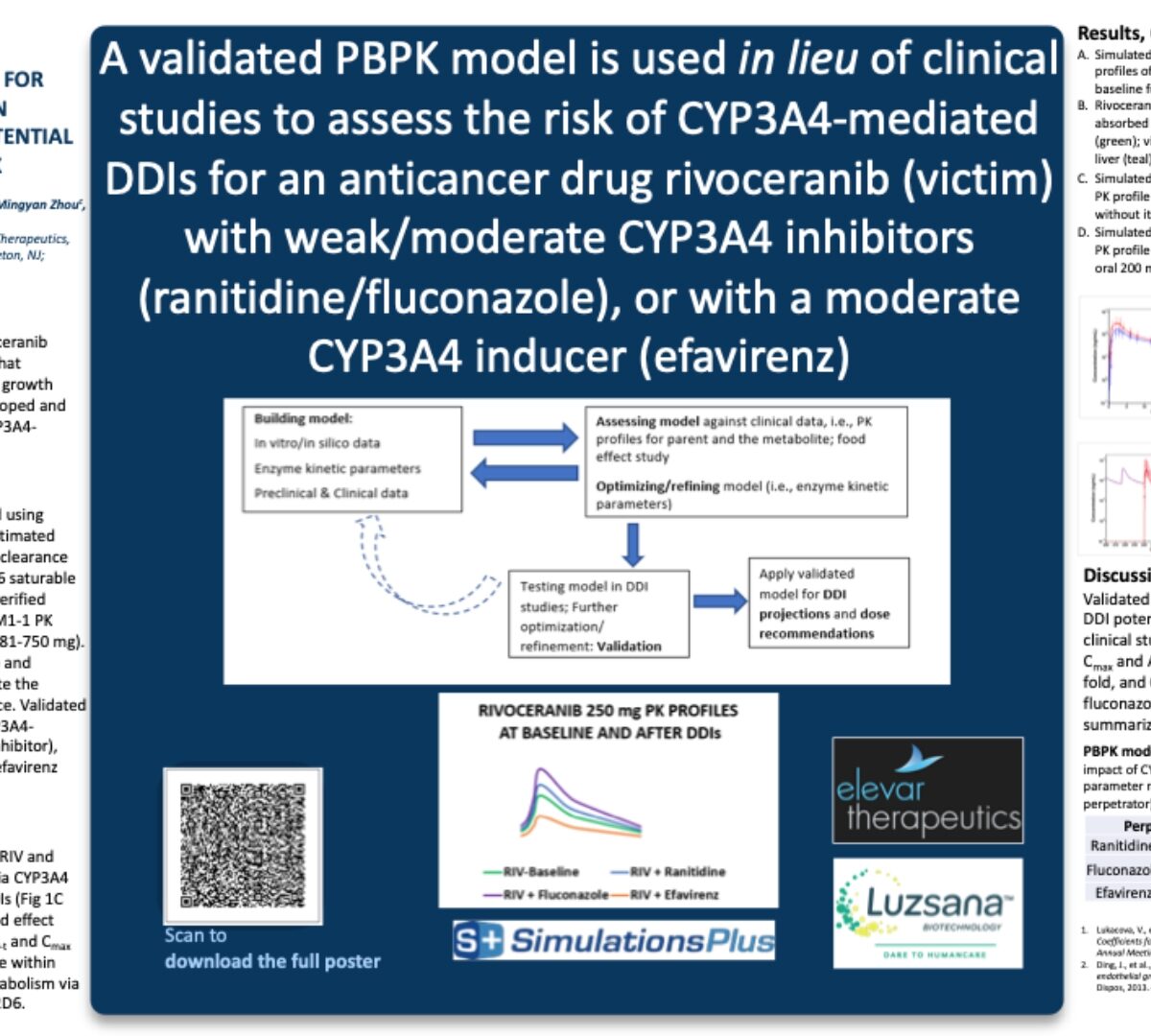
A validated PBPK model is used in lieu of clinical studies to assess the risk of CYP3A4-mediated DDIs for an anticancer drug rivoceranib (victim) with weak/moderate CYP3A4 inhibitors (ranitidine/fluconazole), or with a moderate CYP3A4 inducer (efavirenz)
A PBPK model for anticancer drug rivoceranib (RIV), a tyrosine kinase inhibitor (TKI) that selectively targets vascular endothelia growth factor...

Simulations Plus Sets Date for Second Quarter Fiscal Year 2023 Earnings Release and Conference Call
Conference call to be on Wednesday, April 5, 2023, at 5 p.m. EDT
![CMC development of [14C]-labeled sotorasib for the conduct of microtracer human ADME study](https://www.simulations-plus.com/wp-content/uploads/RC-1080x1080-22.jpg)
CMC development of [14C]-labeled sotorasib for the conduct of microtracer human ADME study
Human absorption, distribution, metabolism, and excretion (hADME) studies of new drugs are required for global regulatory filings.
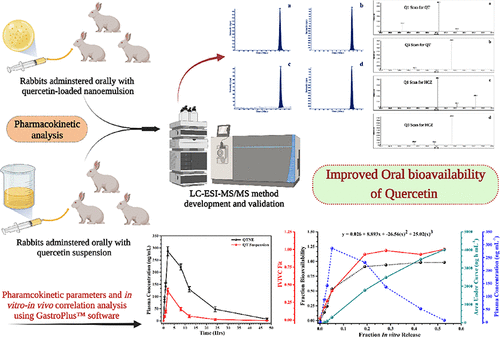
Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry Estimation of Quercetin-Loaded Nanoemulsion in Rabbit Plasma: In Vivo–In Silico Pharmacokinetic Analysis Using GastroPlus
In the present study, we developed and validated a rapid, specific, sensitive, and reproducible liquid chromatography–electrospray ionization tandem mass spectrometry method...
What’s New in MonolixSuite 2023
Discover the new functionalities of MonolixSuite 2023 in this webinar!

Simulations Plus to Present at Sidoti March Small-Cap Conference
Simulations Plus to Present at Sidoti March Small-Cap Conference

Simulations Plus Enters Partnership to Apply AI/ML Technologies to Design Novel Compounds
Promising intellectual property resulting from the collaboration with Polish Academy of Sciences will be jointly owned for further development opportunities

Physiologically Based Pharmacokinetic Modelling to Predict Pharmacokinetics of Enavogliflozin, a Sodium-Dependent Glucose Transporter 2 Inhibitor, in Humans
Enavogliflozin is a sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor approved for clinical use in South Korea.

NAFLDsym v2B Beta Release is Here: Insight into the Next Gen QSP Software Tool in Julia with Blazing Speed and Updated Interfacing
Join us in this webinar to learn about the technical advances made to the NAFLDsym platform in the latest release: version 2B Beta.

March 2023 GastroPlus Newsletter
Daylight savings time is back, so spring and warm weather are not far off!

Computational Analysis and Experimental Testing of the Molecular Mode of Action of Gatastatin and Its Derivatives
Given its critical role in cell mitosis, the tubulin chain represents a viable chemotherapeutic target to solve the specificity issues associated with targeting...

Lixoft Adds New Data Formatting and Reporting Modules to MonolixSuite(TM) in Version 2023R1
Update includes enhanced PKanalix, Monolix, and Simulx modules

March 2023 News/Events
MIDD+ 2023 sessions are now available for playback in our Resource Center