New Version Further Improves Powerful Data Mining and Drug Design Capabilities

Justification of biowaiver for carbamazepine, a low soluble high permeable compound, in solid dosage forms based on IVIVC and gastrointestinal simulation
The aim of the present study was to use gastrointestinal simulation technology and in vitro-in vivo correlation (IVIVC) as tools to investigate a possible extension of biowaiver criteria...

Analysis of Risk Factors in Human Bioequivalence Study That Incur Bioinequivalence of Oral Drug Products
In the study of human bioequivalence (BE), newly developed oral products sometimes fail to prove BE with a reference product due to the high variability in pharmacokinetic (PK)...

Busting the Black Box Myth: Designing Out Unwanted ADMET Properties with Machine Learning Approaches
Drug design is usually understood as “an inventive process of finding new medications based on the knowledge of the biological target” – according to the...

Omeprazole: Physiologically Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-Drug Interactions (DDI)
To optimize a PBPK model of omeprazole for prediction of DDIs with respect to polymorphic expression of CYP enzymes. Omeprazole absorption and pharmacokinetics were simulated using GastroPlus™.

Azole Antifungals: Physiologically-Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-Drug Interactions (DDIs)
Develop PBPK models for azole antifungals for prediction of DDIs. The absorption and pharmacokinetics of azole antifungals were simulated using GastroPlus™. The program's Advanced Compartmental and…

Azole Antifungals: Physiologically-Based Pharmacokinetic (PBPK) Modeling and Prediction of Drug-drug Interactions (DDIs)
Download the poster presented at the Rosenon conference in 2009 on the development of PBPK models for common azole antifungals and DDI predictions

Use of a clinically derived exposure-response relationship to evaluate potential tigecycline-Enterobacteriaceae susceptibility breakpoints
Potential tigecycline-Enterobacteriaceae susceptibility breakpoints were evaluated using 2 approaches, which differed in the nature of the probabilities assessed by MIC value.

Prediction of drug-drug interaction (DDI) between cilostazol and substrates or inhibitors of CYP 2C19 and 3A4
The aim of this study was to validate the utility of physiologically based pharamcokinetic (PBPK) models fore predictioin of DDI between cilostazol, kectoconazole, omeprazole and quindine.

Understanding the effect of API properties on bioavailability through absorption modeling
Selection of API phase is one of the first decision points in the formulation development process.

Simulations Plus Reports FY2008 Financial Results
Earnings Up 17.7%, Shareholder Equity Up 29.4% Over Previous Fiscal Year

Prediction of Drug Clearance by Glucuronidation from in Vitro Data: Use of Combined Cytochrome P450 and UDP-Glucuronosyltransferase Cofactors in Alamethicin-Activated Human Liver Microsomes
Glucuronidation via UDP-glucuronosyltransferase (UGT) is an increasingly important clearance pathway.

Toward an improved prediction of human in vivo brain penetration
The penetration of drugs into the central nervous system is a composite of both the rate of drug uptake across the blood–brain barrier and the extent of distribution into brain tissue compartments.
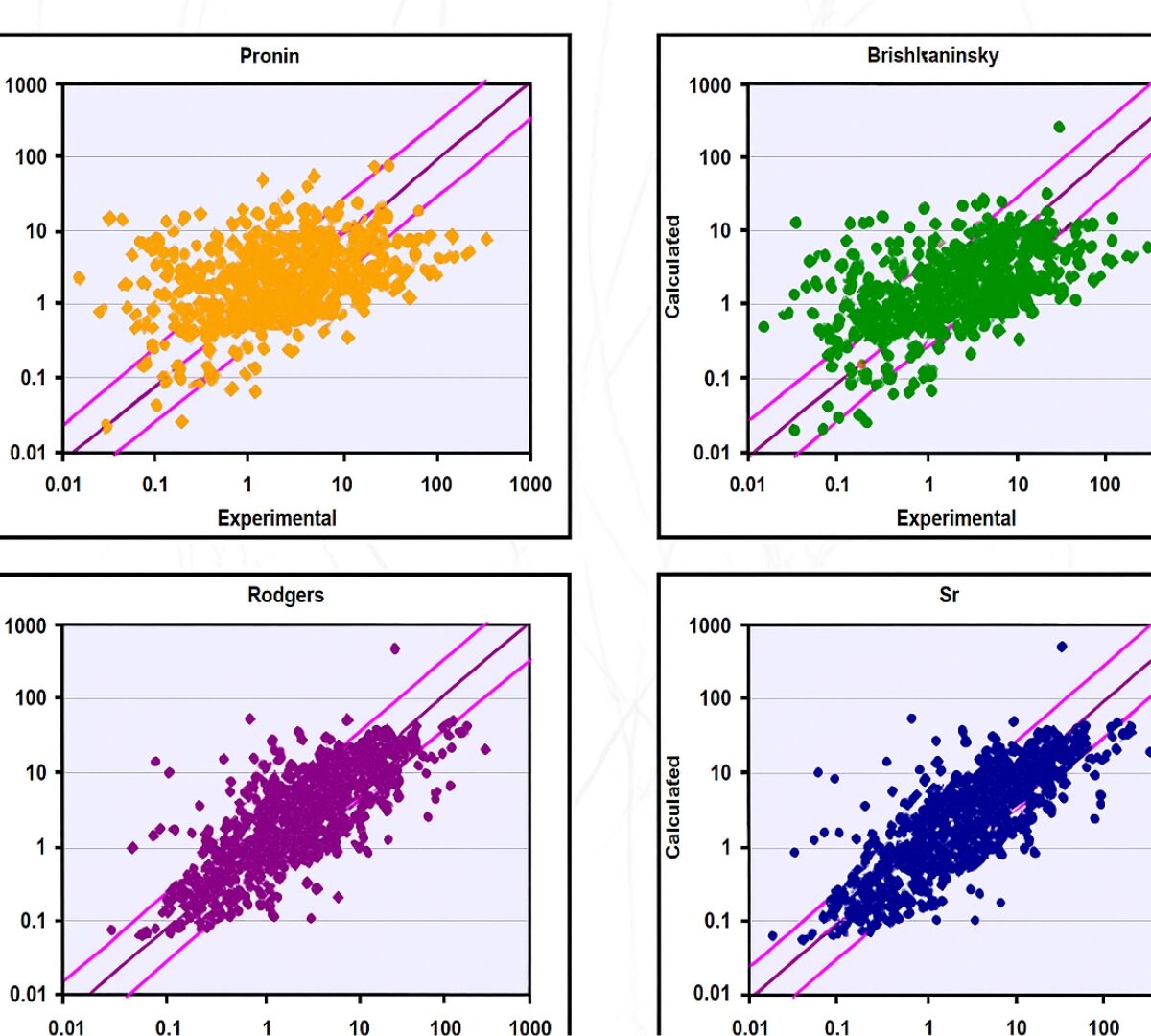
General Approach to Calculation of Tissue:Plasma Partition Coefficients for Physiologically Based Pharmacokinetic (PBPK) Modeling
To conduct a comprehensive evaluation of methods for calculation of tissue/plasma partition coefficients with a focus on correct prediction of volume of distribution and recommendation for a general…

Role of Fraction Unbound in Plasma in Calculations of Tissue:Plasma Partition Coefficients
Previous investigations have shown that the Rodgers and Rowland method [Rodgers 2007] for prediction of tissue:plasma partition coefficients (Kps) provides good prediction for compounds with low to moderate…

Level A IVIVC Using a Comprehensive Absorption/PBPK Model for Metoprolol
Wagner-Nelson, Loo-Riegelman, numerical deconvolution, and convolution-based methods are conventional ways to form an in vitro-in vivo correlation (IVIVC). The ultimate goal for forming an IVIVC is to…

Beauty and the Beast?
Several large Pharma companies have announced interest in acquiring small biotech companies. Many Pharma companies have reduced or eliminated drug discovery efforts, and with stock prices back at 2003 levels, there certainly is a great deal of sense in these acquisitions. But finding another way to integrate these companies and their development portfolio also makes a great deal of sense.

John Muir (1838 to 1914) and advice for surviving the Economic Crisis of 2008
John Muir was one of the first climbers to explore and climb many of the peaks in Yosemite Valley in California’s High Sierra. During his first ascent of Mount Ritter in 1872, he became gripped with fear.
