The liquid chromatographic behavior observed under bimodal retention conditions (reversed phase and hydrophilic interaction) offers a new basis for the determination of some derived lipophilicity indices.

Extemporaneously prepared controlled release formulations for accelerating the early phase development of drug candidates
Extemporaneous drug preparations, which are compounded by a pharmacist at a clinical site, are commonly used in early clinical studies to evaluate the performance of drug candidates.

A PPAR-ß/d Agonist is Neuroprotective and Decreases Cognitive Impairment in a Rodent Model of Parkinson’s Disease
Parkinson's disease (PD) is associated with higher risk of cognitive impairment that may lead to memory loss, confusion, and decreased attention span.

The paradox of scientific excellence and the search for productivity in pharmaceutical R&D
Scientific advances in specialty areas are proceeding at a rapid rate, but the research and development enterprise seems unable to take full advantage. Harnessing the steady stream of knowledge and inventions from different...

Simian Virus 40 Large T Antigen Induces IFN-Stimulated Genes through ATR Kinase
Polyomaviruses encode a large T Ag (LT), a multifunctional protein essential for the regulation of both viral and host cell gene expression and productive viral infection.

Simulations Plus Announces Quarterly Cash Dividend
Cash dividend declared of $0.05 per share

Impact of Delayed-Dose Administration of USL255, Qudexy™ XR (Topiramate) Extended-Release Capsules
Despite the importance of medication adherence for successful management of seizure disorders, nonadherence continues to be a significant problem in patients with epilepsy.

Use of a systems model of drug-induced liver injury (DILIsym(®)) to elucidate the mechanistic differences between acetaminophen and its less-toxic isomer, AMAP, in mice.
Acetaminophen (APAP) has been used as a probe drug to investigate drug-induced liver injury (DILI). In mice, 3'-hydroxyacetanilide (AMAP), a less-toxic isomer of APAP, has also been studied as a negative control.

Simulations Plus Reports Second Quarter FY2014 Financial Results
Company completes 26th consecutive profitable quarter

Simulations Plus Sets Date for 2nd Quarter 2014 Earnings Release and Conference Call
Conference Call to be on Wednesday, April 9, at 4:15 PM ET

From Bench to Humans: Formulation Development of a Poorly Water Soluble Drug to Mitigate Food Effect
This study presents a formulation approach that was shown to mitigate the dramatic food effect observed for a BCS Class II drug. In vitro (dissolution), in vivo (dog), and in silico (GastroPlus®) models...

Application of Physiologically Based Absorption Modeling to Formulation Development of a Low Solubility, Low Permeability Weak Base: Mechanistic Investigation of Food Effect
Physiologically based pharmacokinetic (PBPK) modeling has been broadly used to facilitate drug development, hereby we developed a PBPK model to systematically investigate the...

A Case Study of In Silico Modelling of Ciprofloxacin Hydrochloride / Metallic Compound Interactions
With the development of physiologically based absorption models, there is an increased scientific and regulatory interest in in silico modelling and simulation of drug-drug and drug-food interactions.

Modelling the Absorption of Metformin with Patients Post Gastric Bypass Surgery
Gastric bypass surgery in obesity shortens the length of the small intestine, which can have a significant impact on drug absorption.

Physiologically based pharmacokinetic modeling framework for quantitative prediction of an herb-drug interaction
Herb-drug interaction predictions remain challenging. Physiologically based pharmacokinetic (PBPK) modeling was used to improve prediction accuracy of potential herb-drug interactions...
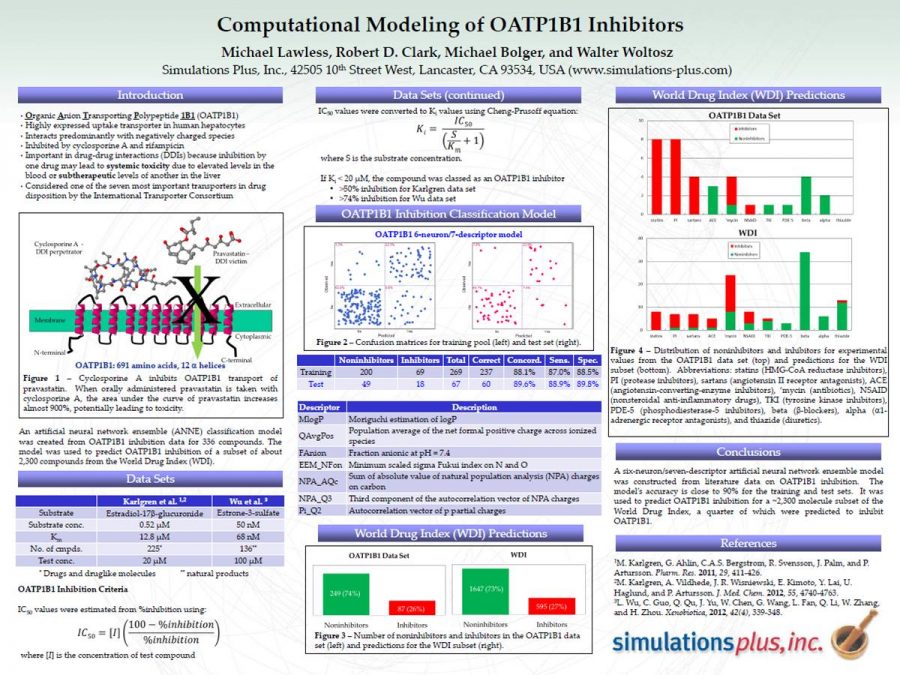
Computational Modeling of OATP1B1 Inhibitors
This poster was presented at the 2014 SOT meeting. It describes where the OATP1b1 data originated and the cutoff value. The model, including descriptors, is discussed.

Quantitative Modeling Uncovers a Potential Limitation in the Putative Mechanism of CCl4 Hepatotoxicity
Drug-induced liver injury (DILI) is one of the leading causes of drug development failures and drug withdrawals. DILIsym® is being developed to identify and mitigate DILI risk through in silico…

Differential effects of RUNX2 on the androgen receptor in prostate cancer: synergistic stimulation of a gene set exemplified by SNAI2 and subsequent invasiveness
Changes to androgen signaling during prostate carcinogenesis are associated with both inhibition of cellular differentiation and promotion of malignant phenotypes.

Simulations Plus Signs New Partnership Agreement in China
Research Institute for Liver Diseases to Offer Company’s Consulting Services

Where top-down meets bottom-up: Combined population PK (PopPk) and PBPK approaches to evaluate the impact of food and gastric pH on the pharmacokinetics of GDC-0941
The phosphoinositide 3-kinase (P13K) signaling pathway is deregulated in a wide variety of cancers. GDC-0941 is a potent and selective pan-inhibitor of class I P13K.
