The aim of this present study was to establish a new in vitro assay, double artificial membrane permeation assay (DAMPA), to evaluate the human intestinal permeability of drugs.

The Poorly Membrane Permeable Antipsychotic Drugs Amisulpride and Sulpiride Are Substrates of the Organic Cation Transporters from the SLC22 Family
Variations in influx transport at the blood-brain barrier might affect the concentration of psychotropic drugs at their site of action and as a consequence might alter therapy response.

Systems pharmacology modeling predicts delayed presentation and species differences in bile acid-mediated troglitazone hepatotoxicity.
Troglitazone (TGZ) causes delayed, life-threatening drug-induced liver injury in some patients but was not hepatotoxic in rats.

Experimental and Computational Prediction of Glass Transition Temperature of Drugs
Glass transition temperature (Tg) is an important inherent property of an amorphous solid material which is usually determined experimentally.

Formulation of the Microbicide INP0341 for In Vivo Protection against a Vaginal Challenge by Chlamydia trachomatis
The salicylidene acylhydrazide (SA) compounds have exhibited promising microbicidal properties.

Simulations Plus Announces Quarterly Cash Dividend of $0.05 Per Share
Cash dividend yield of 3.3% to be distributed in November

Improving genetic programming for the prediction of pharmacokinetic parameters
The prediction of pharmacokinetic parameters is a crucial phase of the drug discovery process, and the automatization of this task is a hot topic in computational bio-medicine.

Simulations Plus Adds to Portfolio with Release of MembranePlus
First Release of New Product in 10 Years; Provides Unique In Vitro Permeability Capabilities and Creates Cross-Selling Opportunities

Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors
The present study aimed to develop nanocrystal formulations of meloxicam (MEL) in order to enhance its biopharmaceutical properties and provide a rapid onset of action. Nanocrystal formulations were...

Systems Pharmacology Modeling Predicts Hepatotoxic Potential Of Troglitazone And Pioglitazone
Troglitazone (TGZ) caused life-threatening drug-induced liver injury (DILI) in diabetic patients, whereas the next in class, pioglitazone (PGZ), has rarely been associated with DILI. Inhibition of bile acid...
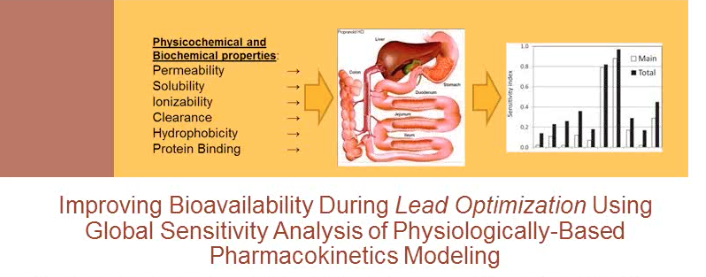
Improving Bioavailability During Lead Optimization
In this presentation, Dr. Eric Martin of Novartis discusses improving bioavailability during lead optimization using global sensitivity analysis (GSA) of physiologically based pharmacokinetics.

Synthesis of a novel universal opioid receptor agonist with the 1,3,5-trioxazatriquinane skeleton and its pharmacologies
We designed and synthesized of 1,3,5-trioxazatriquinanes with o- or p-hydroxyphenyl rings as analogs of the κ opioid receptor agonist SYK-146 with m-hydroxyphenyl groups.

Biopharmaceutical profiling of new antitumor pyrazole derivatives
Several new pyrazole derivatives have demonstrated promising antiproliferative and cytotoxic effects, but their poor solubility raised concerns over possible biopharmaceutical limitations.

Pharma of the Future Historical Timeline
View our milestones.

History of Pharma of the Future
Pharmacometric modeling and simulation has moved from its infancy as a novel way of approaching the analysis of clinical pharmacokinetic data to become an invaluable tool in pharmaceutical and biotechnology research and development.

Pazopanib as third line therapy for metastatic renal cell carcinoma: clinical efficacy and temporal analysis of cytokine profile
Purpose: Pazopanib has been assessed primarily in cytokine refractory or treatment naïve patients with metastatic renal cell carcinoma. Outcomes have been associated...

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Clinical evidence indicated that effective substitution of azithromycin capsule with a tablet dosage form should be based on evidence of providing equivalent in vivo AUC/MIC ratio at the site of infection.

Isoxazolotacrines as non-toxic and selective butyrylcholinesterase inhibitors for Alzheimer’s disease
Owing to the complex nature of Alzheimer's disease, there is a renewed and growing search for multitarget non-toxictacrines as simple, easily available drugs in order to stop the progress and development of the disease.

PEGylated cyclodextrins as novel siRNA nanosystems: Correlations between polyethylene glycol length and nanoparticle stability
Silencing disease-related genes in the central nervous system (CNS) using short interfering RNA (siRNA) holds great promise for treating neurological disorders.
