In pediatric PBPK models, age-related changes in the body are known to occur.

Evaluation of blood-brain barrier penetration and examination of binding to human serum albumin of 7-O-arylpiperazinylcoumarins as potential antipsychotic agents
The delivery of drugs to the brain is complicated by the multiple factors including low blood–brain barrier (BBB) passive permeability, active BBB efflux systems, and plasma protein binding.

Use of a Quantitative Systems Pharmacology (QSP) Model to Predict Liver Toxicity in Simulated Populations
DILIsym is a mechanistic, mathematical model that has been constructed to support pharmaceutical risk assessment and decision making.

8-Hydroxy-2-(1H-1,2,3-triazol-1-yl)-1,4-naphtoquinone derivatives inhibited P2X7 Receptor-Induced dye uptake into murine Macrophages
Extracellular adenosine 5′-triphosphate (ATP) triggers the P2X7 receptor (P2X7R) ionic channel to stimulate the release of the interleukin-IL-1β cytokine into macrophages.

Computational Methods and Tools to Predict Cytochrome P450 Metabolism for Drug Discovery
In this review we present important, recent developments in the computational prediction of cytochrome P450 (CYP) metabolism in the context of drug discovery.

Physiologically based absorption modeling to predict bioequivalence of controlled release and immediate release oral products
Physiologically based absorption modeling was conducted to predict bioequivalence (BE) for immediate release (IR) and controlled release (CR) formulations.

The Optimization Module in GastroPlus®
This tutorial discusses the Optimization module in GastroPlus that allows one to optimize simulation model parameters such as initial dose, Vmax, enzyme expression levels, and many others, in order to...

Synthesis and Biological Evaluation of Novel Triple-Modified Colchicine Derivatives as Potent Tubulin-Targeting Anticancer Agents
Specific modifications of colchicine followed by synthesis of its analogues have been tested in vitro with the objective of lowering colchicine toxicity.

Tools and resources for metabolomics research community: A 2017–2018 update
The scale at which MS‐ and NMR‐based platforms generate metabolomics datasets for both research, core, and clinical facilities to address challenges in the various sciences—ranging from biomedical to agricultural—is underappreciated.

Simulations Plus Reports FY2018 and Fourth Quarter FY2018 Financial Results
Simulations Plus today reported results for the 4th quarter and fiscal year 2018. FY18 revenues increased 23%; earnings per share increased 51.4%.
Full Fiscal Year Pharmaceutical Software and Services Revenues Up 22.9%; Earnings per share of $0.50, up 51.4% over prior year

Bioequivalence comparison of pediatric Dasatinib formulations and elucidation of absorption mechanisms through integrated PBPK modeling
Sprycel® (Dasatinib) is a BCS II weakly basic drug that exhibits strong pH dependent solubility.

A Rapid Method to Estimate Hepatocyte Loss Due to Drug‐Induced Liver Injury
It is not currently possible to rapidly estimate the extent of hepatocyte loss during drug‐induced liver injury (DILI). We used a proprietary mechanistic model (DILIsym) to estimate percentage hepatocyte loss due...

Pharmacokinetics, tissue distribution and excretion of ACT001 in Sprague-Dawley rats and metabolism of ACT001
This study investigated pharmacokinetics, tissue distribution and excretion of ACT001 in Sprague-Dawley rats. Stability study and metabolism study of ACT001 are conducted.

Zonal Hepatic Stellate Cell (HSC) Activation in Nonalcoholic Steatohepatitis (NASH) Characterized by A Mathematical Model
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of pathophysiology, ranging from hepatic steatosis, through non-alcoholic steatohepatitis (NASH) and hepatic fibrosis, and in rare cases resulting in cirrhosis and liver failure.

Quantitative Systems Toxicology (QST) Supports Differentiated Liver Safety for a Next-in-Class Compound
Lixivaptan, a vasopressin-2 receptor antagonist, is under development for the treatment of autosomal dominant polycystic kidney disease (ADPKD), an orphan disease with minimal treatment options.
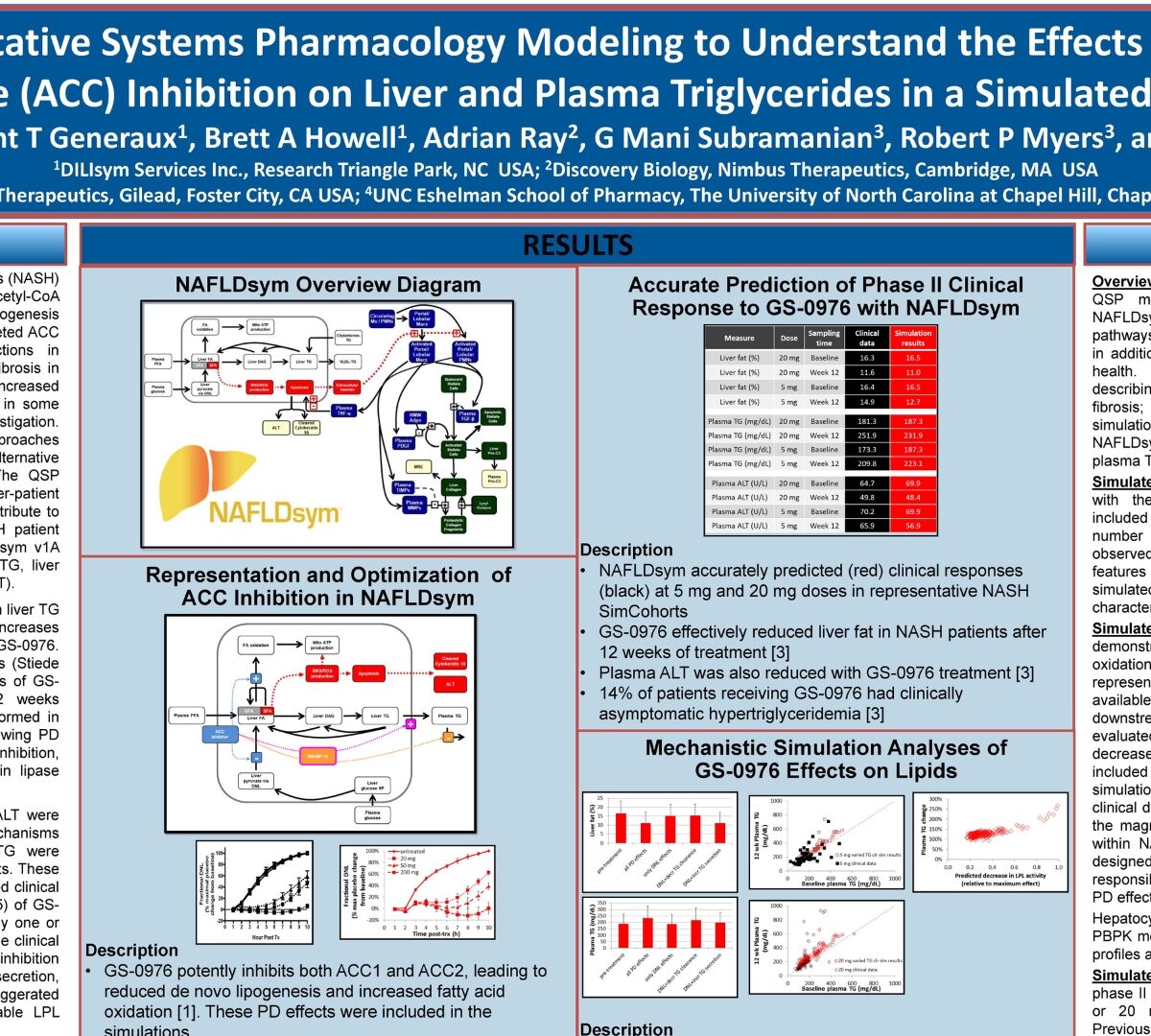
Using Quantitative Systems Pharmacology Modeling to Understand the Effects of Acetyl CoA Carboxylase (ACC) Inhibition on Liver and Plasma Triglycerides in a Simulated Population
Treatment options for nonalcoholic steatohepatitis (NASH) are limited. One approach targets hepatic acetyl-CoA carboxylase (ACC), which influences de novo lipogenesis (DNL) and fatty acid oxidation.

In Vitro to In Vivo Extrapolation (IVIVE) of Itraconazole Precipitation using a Biphasic Dissolution Test and Mechanistic Absorption Model
Regulatory agencies have encouraged the use of mechanistic absorption (MAM) and physiologically-based pharmacokinetic (PBPK) modeling to reduce cost and time to market for new and generic drug products.

A Physiologically Based Pharmacokinetic Model of Rivaroxaban: Role of OAT3 and P-gp Transporters in Renal Clearance
Rivaroxaban is an oral anticoagulant which acts by inhibiting factor Xa of the coagulation network.
