For performance assessment of the lipid-based drug delivery systems (LBDDS), in vitrolipolysis is commonly applied because traditional dissolution tests do not reflect the complicated in vivo micellar formation and solubilisation processes.

Model-based drug development in pulmonary delivery: Pharmacokinetic analysis of novel drug candidates for treatment of Pseudomonas aeruginosa lung infection
Antibiotic resistance is a major public health threat worldwide. In particular, about 80% of cystic fibrosis patients have chronic Pseudomonas aeruginosa (PA) lung infection resistant to many current antibiotics.

Estimating Predictive Uncertainty for Ensemble Regression Models by Gamma Error Analysis
The Standard Error (SE) of Prediction: a Measure of Individual Uncertainties

Galactosylated chitosan Triptolide nanoparticles for overcoming hepatocellular carcinoma: Enhanced therapeutic efficacy, low toxicity, and validated network regulatory mechanisms
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related deaths worldwide. Current therapies present significant limitations.

Dibutyltin(IV) Complexes Derived from L-DOPA: Synthesis, Molecular Docking, Cytotoxic and Antifungal Activity
A series of organotin(IV) complexes was herein prepared and characterized. A one-pot synthetic strategy afforded reasonable to high yields, depending on the nature of the ligand.

Bringing physiologically-based pharmacokinetic (PBPK) simulation to early drug discovery and development
Lack of efficacy continues to be a problem in drug development. Piecemeal rules of thumb such as Lipinski’s Rule of Five [1] help avoid compounds likely to be poorly...

Critical Evaluation of 2-Ethylhexyl Acrylate Dermal Carcinogenicity Studies Using Contemporary Criteria
Skin tumors have been observed in C3H/HeJ mice following treatment with high and strongly irritating concentrations of 2-ethylhexyl acrylate (2-EHA).

Simulations Plus Receives New Grant Award from the FDA
Simulations Plus today announced today a new funded cooperative agreement with the FDA to work on the GastroPlus dermal absorption/PBPK (TCAT) model.

The PKPlus Module in GastroPlus® Tutorial
This video shows how to create noncompartment, 1-, 2-, and 3-compartment pharmacokinetic models based on in vivo data using the PKPlus™ module in GastroPlus.

Computer-aided drug discovery of Myc-Max inhibitors as potential therapeutics for prostate cancer
While Myc is an essential regulator of growth in normal cells, it is also frequently associated with cancer progression, therapy-resistance and lethal outcomes in most human cancers.

In silico scaling and prioritization of chemical disposition and chemical toxicity of 15,145 organic chemicals
This report describes the development and beta-test of methods that prioritize and scale in silico predictions of chemical disposition {(CD) intestinal absorption, membrane permeability...

In Silico Study of Pyrazolylaminoquinazoline Toxicity by Lazar, Protox, and Admet Predictor
Pyrazolylaminoquinazoline is obtained from synthetic AZD4547 and can inhibit kinase activity in recombinant fibroblast growth factor receptor (FGFR) in vitro.

Structural and functional pharmacokinetic analogs for physiologically based pharmacokinetic (PBPK) model evaluation
Physiologically based pharmacokinetic (PBPK) models enable simulations of absorption, distribution, metabolism, and elimination of chemicals from the body.

Virtual screening using covalent docking to find activators for G245S mutant p53
TP53 is the most mutated gene in all cancers. The mutant protein also accumulates in cells. The high frequency of p53 mutations makes the protein a promising target for anti-cancer therapy.

In Vivo Predictive Dissolution and Simulation Workshop Report: Facilitating the Development of Oral Drug Formulation and the Prediction of Oral Bioperformance
A 2-day workshop entitled “In Vivo Predictive Dissolution and Simulation” was held September 11–12, 2017 in Washington, DC, focused on the selection of applications, methodologies, and...

Exploring pharmacological mechanisms of Xueshuan-Xinmai-Ning tablets acting on coronary heart disease based on drug target-disease gene interaction network
Xueshuan-Xinmai-Ning Tablet (XXNT), a commercially available patent drug, has been extensively used in the treatment of coronary heart disease (CHD) with a satisfying therapeutic efficacy.

U.S. FDA Extends Funded Research Collaboration with Simulations Plus
Simulations Plus announced today that it received an extension to its funded research collaboration with the FDA to develop the GastroPlus OCAT model.

Study of degradation behaviour of montelukast sodium and its marketed formulation in oxidative and accelerated test conditions and prediction of physicochemical and ADMET properties of its degradation products using ADMET Predictor™
Study of oxidative stability of pharmaceutical actives and formulations is important as oxidation pathway is the second most significant route for the decay of pharmaceuticals.

Computational screening of known broad-spectrum antiviral small organic molecules for potential influenza HA stem inhibitors
With the emergence of new influenza virus strains that are resistant to current inhibitors such as oseltamivir (anti-neuraminidase (NA)) and amantadine (anti-M2 proton channel), influenza A viruses...
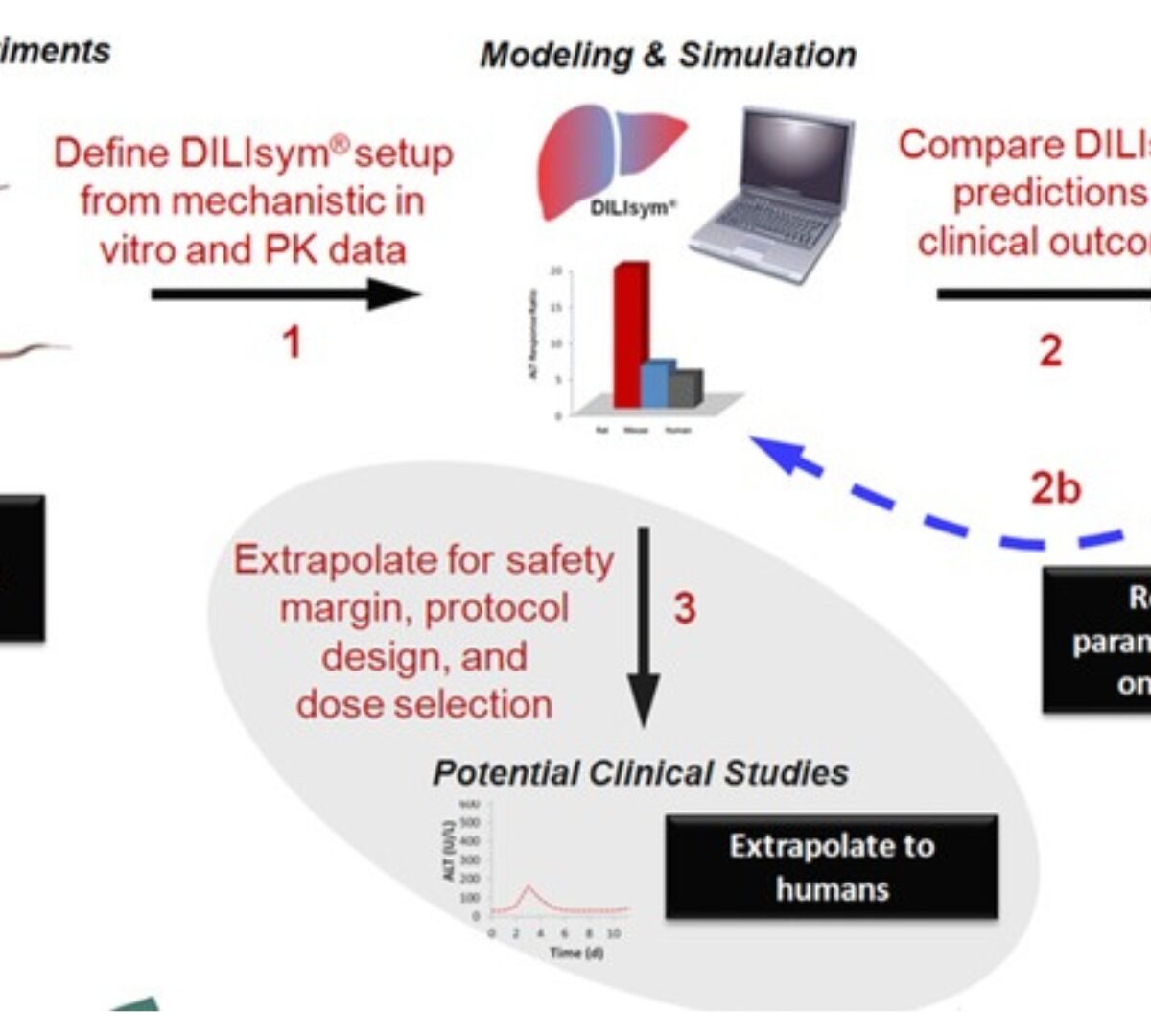
Prediction of Safety Margin and Optimization of Dosing Protocol for a Novel Antibiotic using Quantitative Systems Pharmacology Modeling
Elevations of liver enzymes have been observed in clinical trials with BAL30072, a novel antibiotic. In vitro assays have identified potential mechanisms for the observed hepatotoxicity, including...
