Due to their unique application and action, inhalation products require specific quality tests, such as Uniformity of Delivered Dose and Aerodynamic Assessment of Fine Particles.

Thermodynamics-Informed Neural Networks and Extensive Data Sets: Key Factors to Accurate Blind Predictions of Apparent pKa Values in the EuroSAMPL Challenge
Microscopic and macroscopic pKa values for 35 compounds selected by the organizers of euroSAMPL 1 challenge were blindly predicted with our thermodynamics-informed empirical S + pKa model (ranked submission 0x4cb7101f).

Anxiety Disorders, PTSD and OCD: Systematic Review of Approved Psychiatric Medications (2008–2024) and Pipeline Phase III Medications
This systematic review examines psychiatric medications approved by the FDA for anxiety disorders, post-traumatic stress disorder (PTSD) and obsessive-compulsive disorder (OCD) from 2008 to 2024 and describes the mechanism of action, indications for both labelled and off-label uses, evidence for efficacy, dosing and adverse effects for each medication.

Simulations Plus Reports Second Quarter Fiscal 2025 Financial Results
Total revenue grew 23% year-over-year driven by strong growth in both software and services

Advances in Physiologically Based Pharmacokinetic (PBPK) Modeling and its Regulatory Utility to Support Oral Drug Product Development and Harmonization
This report summarizes the proceedings of Session 1 of the one-day public workshop titled “Advances in PBPK Modeling and its Regulatory Utility for Oral Drug Product Development” hosted by the U.S. Food and Drug Administration (FDA) and the Center for Research on Complex Generics (CRCG) on October 12, 2023.

High vs. Low Vancomycin Therapeutic Concentrations in Periprosthetic Joint Infection: A Retrospective Cohort Analysis
Current guidelines recommend vancomycin concentrations of 10–20 μg/mL for most infections, with higher levels (15–20 μg/mL) suggested for severe cases.
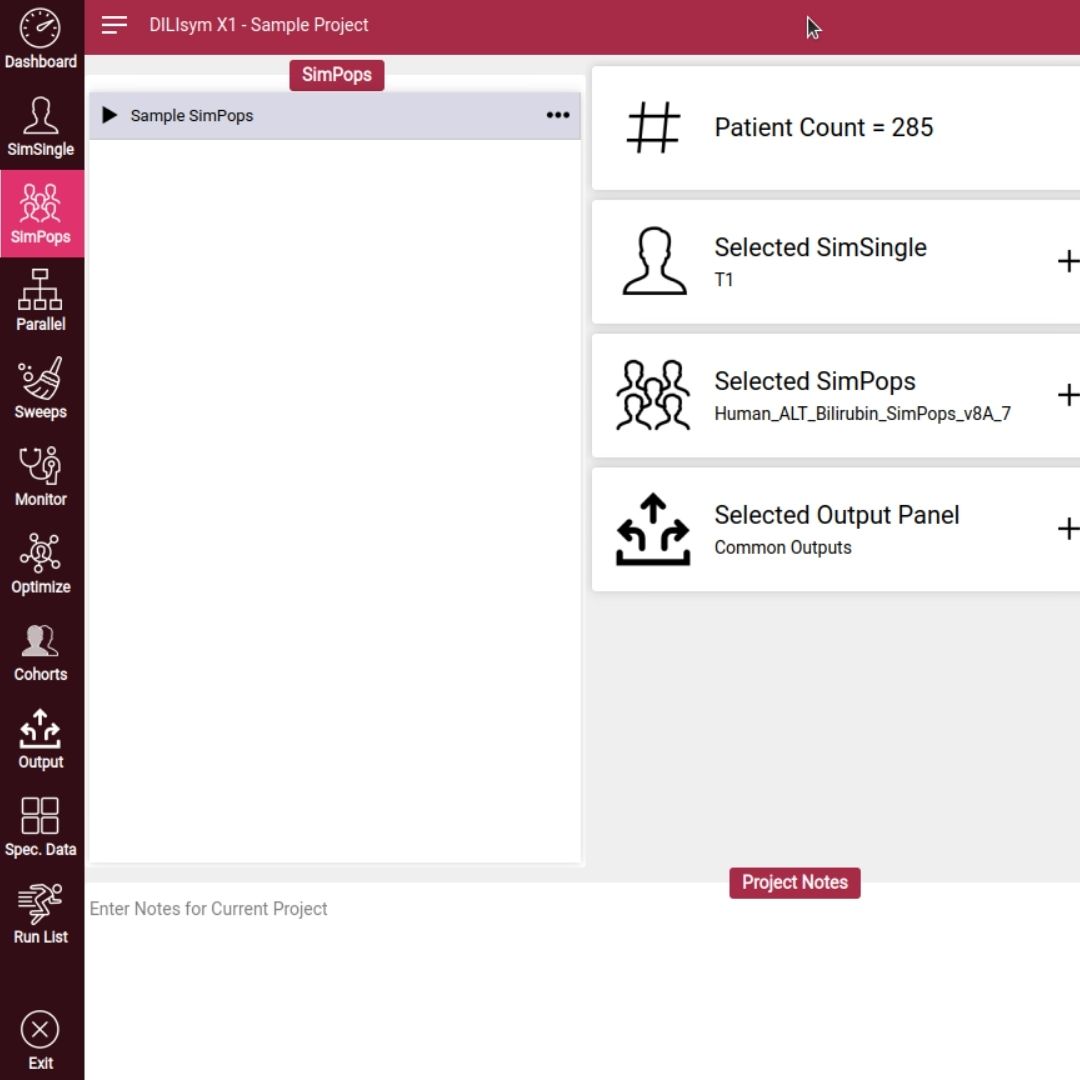
Accelerating Small Molecule GLP-1 Agonist Optimization: AI-Driven Design Meets Mechanistic Modeling for a First-to-Invent Advantage
In a fast-paced industry with big budget competition, you need every tool and approach to succeed.

PBPK Modeling to Support Bioavailability and Bioequivalence Assessment in Pediatric Populations
This report summarizes the proceedings for Session 3 of the one-day public workshop entitled “Advances in PBPK Modeling and its Regulatory Utility for Oral Drug Product Development” a jointly sponsored workshop by U.S. Food and Drug Administration (FDA) and the Center for Research on Complex Generics (CRCG) on October 12, 2023.

In Vitro and In Vivo Pharmacokinetic Characterization of 7-Hydroxymitragynine, an Active Metabolite of Mitragynine, in Sprague-Dawley Rats
Kratom, a Southeast Asian tree, has been researched for its potential as a therapeutic for substance use disorders.

Antiviral Activity of Halogenated Compounds Derived from L-Tyrosine Against SARS-CoV-2
Currently, there are no effective medications for treating all the clinical conditions of patients with COVID-19.

Trends in Drug-Drug Interactions for New Drug Clinical Trials in China Over the Past 10 Years (2013–2022)
The number of drug-drug interaction (DDI) clinical trials in China has increased rapidly in recent years.

Unveiling the Phenolic Profiling of Moroccan Ferula communis L. Fruits: A Combination of In Silico and In Vivo Protective Effect Against Methotrexate-Induced Hepato-Renal Dysfunction
Methotrexate (MTX) is associated with several side effects, including hepatic and renal toxicities, which limit its effectiveness as an anticancer medication.

Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives
Schistosomiasis is caused by flatworms of the genus Schistosoma, for which mollusks of the genus Biomphalaria are intermediate hosts.
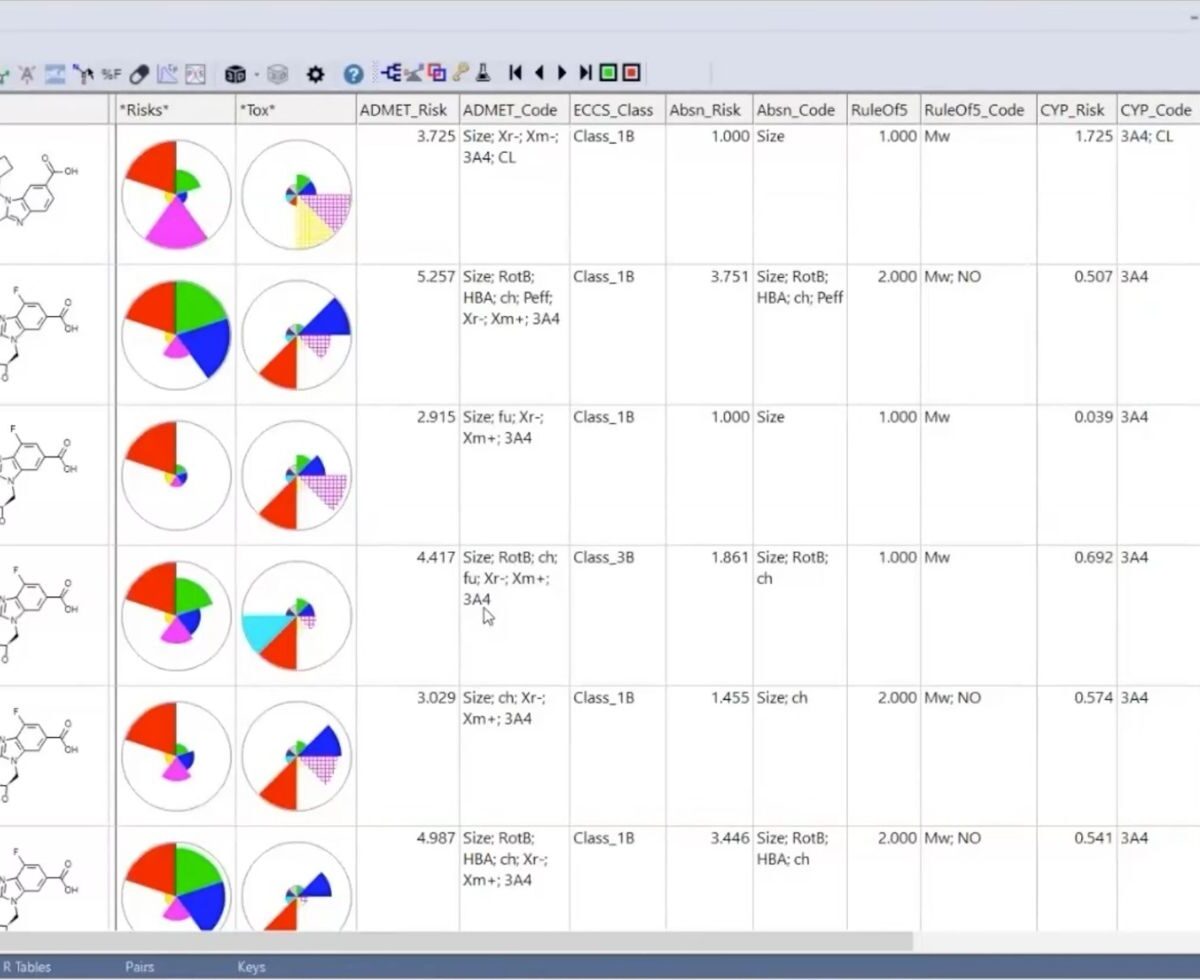
Longitudinal Model-Based Meta-Analysis (MBMA) Comprehensive MonolixSuite Tutorial with Case Studies
Model-based meta analysis (MBMA) informs key drug development decisions by integrating data, published or unpublished, from multiple studies.

Clustering of Environmental Compounds Based on Structure and Toxicokinetic Properties
Traditional toxicokinetic (TK) models rely heavily on in vivo data, necessitating animal testing. At the same time, the scientific toolbox is expanding with new approach methodologies (NAMs) that do not rely on TK studies.

Leveraging Omeprazole PBPK/PD Modeling to Inform Drug–Drug Interactions and Specific Recommendations for Pediatric Labeling
Omeprazole is widely used for managing gastrointestinal disorders like GERD, ulcers, and H. pylori infections.

Physiologically Based Pharmacokinetic Models for Infliximab, Ipilimumab, and Nivolumab Developed with GastroPlus to Predict Hepatic Concentrations
Infliximab, ipilimumab, and nivolumab are three monoclonal antibodies that have been associated with hepatotoxicity.
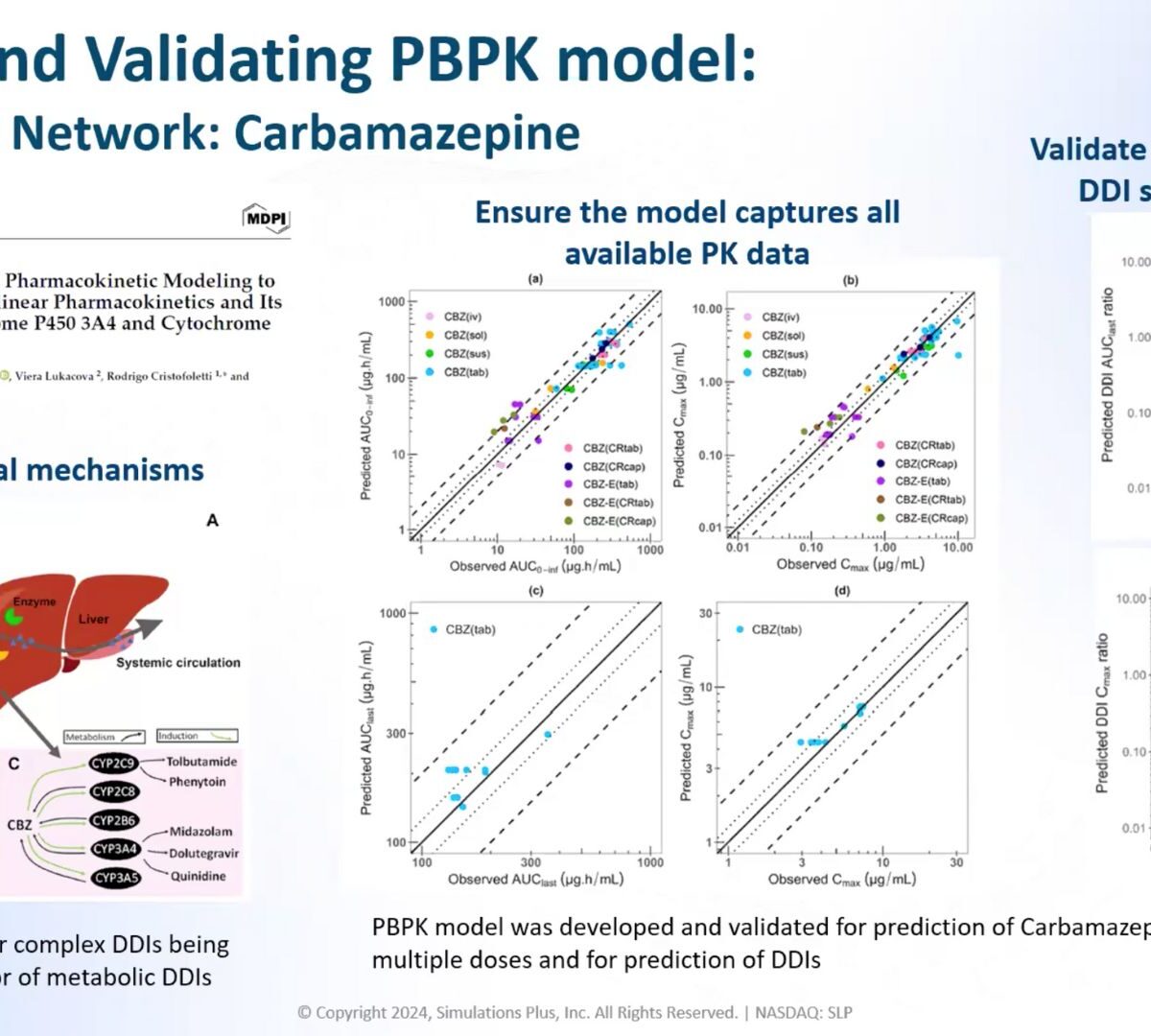
Mastering DDI Risk Assessment: Predict CYP enzyme-mediated DDIs with confidence
Understanding and predicting drug-drug interactions (DDIs) is crucial for ensuring patient safety and improving drug product development strategies.