This letter describes a focused, multi-dimensional optimization campaign around BL-1249, a fenamate class non-steroidal anti-inflammatory and a known activator of the K2P potassium channels TREK-1 (K2P2.1) and TREK-2 (K2P10.1).

Computational Model To Predict the Fraction of Unbound Drug in the Brain
Knowing the value of the unbound drug fraction in the brain (fu,brain) is essential in estimating its effects and toxicity on the central nervous system (CNS)...

Novel isoxazole derivatives as potential antiparkinson agents: synthesis, evaluation of monoamine oxidase inhibitory activity and docking studies
Selective monoamine oxidase B inhibitors are potential drug candidates for the treatment of Parkinson’s disease.

Computational Systems Pharmacology-Target Mapping for Fentanyl-Laced Cocaine Overdose
The United States of America is fighting against one of its worst-ever drug crises.

DILIsym Releases NAFLDsym® Version 2A
Substantial update of NAFLD drug efficacy software offers a broad range of pathophysiology and over a thousand simulated patients for testing

In-silico Approaches to Study Therapeutic Efficacy of Nutraceuticals
Medicinal plants are sources of bioactive compounds for curing ailments since ages.

Requirements to Establishing Confidence in Physiologically Based Pharmacokinetic (PBPK) Models and Overcoming Some of the Challenges to Meeting Them
When scientifically well-founded, the mechanistic basis of physiologically based pharmacokinetic (PBPK) models can help reduce the uncertainty and increase confidence in extrapolations outside the studied scenarios or studied populations.

In Silico Prediction of Isoliquiritigenin and Oxyresveratrol Compounds to BCL-2 dan VEGF-2 Receptors
Isoliquiritigenin and oxyresveratrol are compounds that have been reported to have anticancer activities.

Overview of Brazilian Requirements for Therapeutic Equivalence of Orally Inhaled and Nasal Drug Products
Brazil has established a framework for provision of generic pharmaceuticals including for orally inhaled and nasal drug products (OINDP) to its populace.

Stability behaviour of antiretroviral drugs and their combinations. 10: LC-HRMS, LC-MSn, LC-NMR and NMR characterization of fosamprenavir degradation products and in silico determination of their ADMET properties
The present study focused upon the forced degradation behaviour of fosamprenavir (FPV), an antiretroviral drug. A total of six degradation products (DPs) were separated on a non-polar stationary phase by high performance liquid chromatography (HPLC).

Impact of Intracellular Concentrations on Metabolic Drug-Drug Interaction Studies
Accurate prediction of drug-drug interactions (DDI) is a challenging task in drug discovery and development. It requires determination of enzyme inhibition in vitro which is highly system-dependent for many compounds.

Lipid profile is associated with decreased fatigue in individuals with progressive multiple sclerosis following a diet-based intervention: results from a pilot study
To investigate associations between lipid profiles and fatigue in a cohort of progressive multiple sclerosis (MS) patients on a diet-based multimodal intervention.

Simulations Plus Upgrades Flagship GastroPlus
User- and collaboration-driven functionality in version 9.7 expands the science of local and systemic exposure

A focus on riociguat in the treatment of pulmonary arterial hypertension
Current treatment of pulmonary arterial hypertension (PAH) targets three signalling pathways: the nitric oxide (NO) pathway, the endothelin pathway and...

What’s Next for Fit-for-Purpose (F4P) Models?
Taking fit-for-purpose (F4P) models to the next level – Collaboration between scientists and software designers

Population pharmacokinetic (PopPK) and concentration-QTc analysis of quizartinib in patients (pts) with FLT3-ITD–positive relapsed/refractory (R/R) acute myeloid leukemia (AML)
Fms-related tyrosine kinase 3 (FLT3) is expressed in hematopoietic progenitor cells;
signaling through FLT3 promotes their proliferation and differentiation. FLT3 is mutated
in approximately 30% of patients with AML.
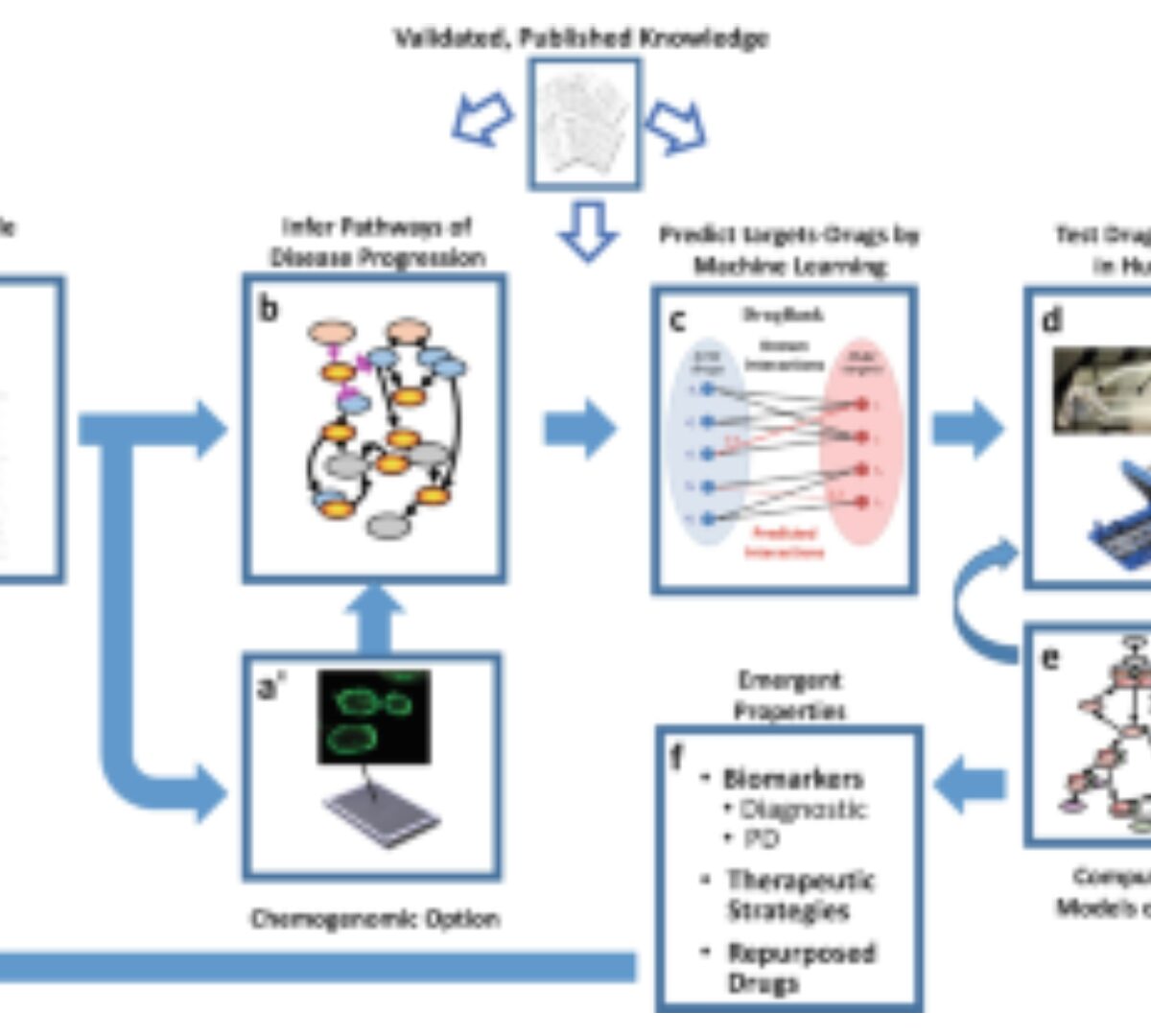
Harnessing Human Microphysiology Systems as Key Experimental Models for Quantitative Systems Pharmacology
Two technologies that have emerged in the last decade offer a new paradigm for modern pharmacology, as well as drug discovery and development.

Different roles of peroxisome proliferator-activated receptor gamma isoforms in prostate cancer
Due to the increasing occurrence of and high costs associated with prostate cancer (PC), there is an urgent need to develop novel PC treatment and chemoprevention...

Uncovering the pharmacological mechanism of Carthamus tinctorius L. on cardiovascular disease by a systems pharmacology approach
Carthamus tinctorius L. is widely used in traditional Chinese medicines for the treatment of cardiovascular disease.

Standalone Installation Instructions for GastroPlus v9.7
Before you begin in order to install GastroPlus, you must have Administrative privileges.
