New guidance was recently released by the International Council for Harmonisation (ICH) regarding bioequivalence for immediate release solid oral dosage forms. While the entire document is worth reading (ICH_M13A_Step4_Final_Guideline_2024_0723.pdf), this blog post summarizes the key points you need to know to waive the new bioequivalence study with proton pump inhibitors.
The ICH Guideline M13A on Bioequivalence for Immediate-Release Solid Oral Dosage Forms in section 3.4 pH Dependency states: “in certain situations, an additional BE assessment with concomitant treatment of a pH-modifying drug product would generally be necessary if all of the following criteria are met:
- The drug products under comparison contain a drug substance with pH-dependent solubility in the pH range of 1.2 – 6.8.
- The product is expected to be taken with acid reducing agents, e.g., proton pump inhibitors, or is going to be used in certain populations, e.g., patients with achlorhydria.
- There are qualitative or quantitative differences in the pH-modifying excipient(s), significant differences in the manufacturing process that may affect drug absorption due to gastric pH differences, or differences in the salt or polymorphic form that possess a different pH-dependent solubility.
This bioequivalence (BE) study involving proton pump inhibitors (PPI) pretreatment to alter stomach pH is a new regulatory requirement. The updated guideline now generalizes this requirement to all active substances with pH-dependent solubility in the pH range of 1.2 to 6.8, encompassing not only bases, which are the most critical, but also acids.
This requirement also applies to test products where the comparator’s formulation avoids pH impact on absorption, and to cases where excipients unintentionally modify pH or different salts or polymorphs have varied pH-dependent solubility. If different salts or polymorphs are used in test and reference products, their solubility-pH profiles must be compared.
What are the opportunities to waive the BE study request?
Applicants can justify the waiver of the BE study under gastric pH-altered conditions. This justification should be based on comprehensive evidence such as the solubility-pH profile of the drug substance, the impact of excipients, formulation, and manufacturing design, differences between the test and comparator products, and comparative dissolution testing at various pH levels. Additionally, validated PBPK absorption models or physiologically based biopharmaceutics modeling (PBBM), and virtual BE simulations, can be used to assess the risk of bioinequivalence.
A waiver of the PPI study could also be possible in the limited cases where the drug product can neither be taken with acid reducing agents nor by patients with achlorhydria (e.g. elderly patients).
It is important to determine if the manufacturing process and material properties impact test product dissolution/absorption under elevated gastric pH conditions. The recommended approach is to show similar dissolution profiles for both products across the pH range that includes the altered gastric pH conditions. These dissolution tests should be discriminative and reflective of the in vivo behavior. Since conventional dissolution media at pH 1.2, 4.5, and 6.8 may not be sufficient,
using additional media is recommended. The 2024 publication from Wu et al. (Drug Metab. Rev. 3:1-20) identifies some biorelevant dissolution testing for weak bases, whilst the 2019 paper from Segregur et al. (J. Pham. Sci. 108(11):3461-3477) describes media mimicking the gastric environment after acid reducing treatment (ARA) administration. If in vitro dissolution profiles of test and reference products differ, a validated PBBM/PBPK model can predict exposure ratios resulting from this dissolution difference.
The larger the number of changes with respect to the comparator product, the more difficult it is to justify that multiple small differences have no impact on the bioavailability in altered gastric pH conditions. A holistic approach based on PBBM/PBPK modeling can integrate all the information on solubility-pH differences due to salt or polymorph differences, pH modified by excipients, and/or dissimilar dissolution profiles at any relevant pH.
The following decision tree summarizes the regulatory strategy to follow:
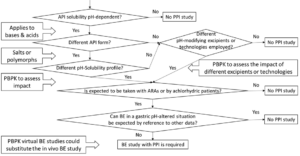
The regulatory acceptance of PBBM/PBPK models with demonstrated predictive ability varies based on the specific scenario and application. A PBBM/PBPK model may show that differences in the API form, the excipients, or the manufacturing method do not impact bioequivalence between test and reference products, even under altered gastric pH conditions.
What criteria should the PBBM/PBPK model meet to accept virtual BE studies in lieu of in vivo BE studies with PPI?
The PBBM should be able to predict the effect of an altered stomach pH on the exposure of formulation variants, and this information could be leveraged to inform the establishment of a dissolution safe space. The variants employed to develop the PBBM should cover the proposed ranges of the product that is to be waived from the in vivo PPI study. The 2020 publication from Mitra et al. (J. Pharm. Sci. 109(3):1380-1394) presented case studies from several pharmaceutical companies demonstrating the utility of PBBM/PBPK modeling and highlighting strategic workflows to inform regulatory decisions in this space.
Key takeaways from this new ICH Guideline requirement
In principle, an additional bioequivalence study with PPI pretreatment is necessary if the proposed product differs from the reference product. This study can easily run $1M USD or more depending on several cost factors (study design and protocol development, clinical site fees, number of participants) and delay filings by several months, which can impact market position and patient access for your new therapy. However, if justified, this additional study can be waived, and PBBM/PBPK modeling is an efficient, proven approach to support this.
If you need PBBM/PBPK modeling expertise to evaluate the effect of PPI on the test and the reference product or designing your regulatory approach, we are here to help.
Want to learn more about regulatory views on BE waivers? Register for our upcoming webinar, Leveraging PBPK/PBBM in Support of BCS Class 3 Biowaivers.
