Patient safety is paramount in any clinical trial. Mistakes in implementing a study protocol—from enrollment, through consenting, through application of novel treatments—can pose unacceptable risks to patient safety. That makes selecting the best training for each program paramount to ensure better safety and better study outcomes.
If training can ensure that researchers make the right decisions every step of the way, patient safety can be dramatically improved. To reach that goal, all members of the research team must understand the protocol inside and out, so they don’t make mistakes when enrolling and treating participants.
There are myriad critical decision points throughout any clinical trial. Errors at any of these points could compromise patient safety. A 2021 study by Tufts CSDD, for instance, notes that protocol complexity has grown steadily since 2009, with the mean number of distinct procedures rising 44%, and will likely continue to do so.

And as trials grow in complexity, that means more decision points and more opportunities for mistakes. The CSDD reported in 2022 that Phase 3 protocols have a mean average of 119 protocol deviations. And the FDA’s most recent BIMO metrics report lists protocol deviations or failure to follow the investigational plan as the most common observations after a BIMO inspection.
The recruitment process offers a good example of a critical decision point. Mistakes in applying inclusion/exclusion criteria can lead to some qualified participants being overlooked. Conversely, some patients may be enrolled in a trial only to find out that they meet some of the exclusion criteria and cannot proceed.
The former situation can hinder full enrollment, while the latter is frustrating for both patients and investigators. Additionally, inclusion of patients that don’t fully meet all criteria could increase the risk to those individuals.
Enhancing Protocol Adherence Through Simulation-Based Training
Simulation-based training, such as the customized modules the Pro-ficiency Performance Management platform offers, allow research staff to practice applying inclusion/exclusion criteria using scenarios that mirror what they will experience when the clinical trial begins. Rather than applying an abstract checklist of criteria, this approach allows research staff to gain experience, make mistakes and receive coaching in the moment to build their decision-making skills.
This deeper understanding developed through the virtual practice also helps practitioners allay any fears patients may have, allowing the healthcare provider to accurately describe potential risks and benefits.
Among researchers trained using the Pro-ficiency modules, for instance, 97.2% reported that the training helped them make more effective decisions, while 94.5% said the training made them feel more confident in implementing the protocol.
And finally, adaptive learning ensures a deep understanding of the protocol so research staff can accurately describe what will be required of the patient for study participation. This plays a major role in how well patients comply with study parameters, which reduces risks to their safety, as well as whether they remain in the trial.
Deviations Reduced with Adaptive Learning
Clinical trials involve use of novel drugs, medical devices and medical procedures, any of which may require clinicians to do things that fall outside their experience in standard care of similar patients. Training that does not ingrain these new procedures sufficiently can increase the risk of deviations, any of which could pose a safety risk to participants.
But when members of a research team are trained on a protocol using simulation, the focus on critical decision points greatly increases the likelihood of the protocol being followed precisely. Mistakes are going to occur, but errors during simulation training can be corrected in the moment, meaning fewer mistakes occur with real patients during the clinical trial.
A particular area where deviations can occur is in administration of the trial drug. Adaptive learning ensures that investigators and research nurses fully understand how to handle, prepare and administer novel drugs during a clinical trial. It also makes clear any potential adverse events. The same can be true in device trials where novel devices and procedures that may differ from standard of care come into play.
Boosting Confidence and Decision-Making with Adaptive Learning
Research staff trained using the Pro-ficiency simulation-based platform, for example, have made 2 million mistakes in the simulated environment, rather than as protocol deviations during an active study. In fact, 100% of studies that utilized the Pro-ficiency platform for training during the design phase at their research sites were able to avoid protocol amendments.
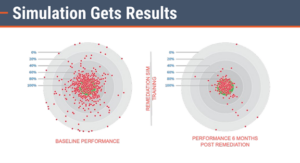
And a 2010 analysis of data from a country-wide deployment in East Africa for the CDC also showed the value of simulation in training clinicians on a rigorous HIV treatment protocol. The image below illustrates the results of that training. The acceptable level of clinical variance from a simulated patient encounter is represented in the green center in the circles below. Red circles indicate an action within an unacceptable level of clinical variance. The analysis showed a pass rate of 69% initially, with an increase to 97% after simulation training.
One example of the value of adaptive learning when novel procedures are involved can be found in a Phase 3 case study, when ProKidney leveraged simulation training to address growing complexities. They were conducting a clinical trial for a novel cell therapy platform that was designed to treat multiple chronic kidney diseases. It involved a therapy using autologous cells, which require careful handling, and a novel delivery system and technique. The combined complexity presented multiple challenges in training various research staff involved in the process —from harvesting of cells, through injection of the therapy into exactly the right place in the patient’s kidney.
The adaptive learning approach ProKidney took allowed researchers to make mistakes in a virtual environment, sharpening their decision-making before applying it to patients. The feedback mechanisms in simulation training like the Pro-ficiency modules help researchers understand mistakes made during training and guide them through reaching the right decision. This is critical with investigative products that are unfamiliar or differ from standard of care for a particular condition or patient subset, and ultimately enhances patient safety and the quality of the clinical trial process.
If you’re interested in diving deeper, click here to book a demo.
By Margaret Richardson, Executive Director, Operations, QMS
