The presentation illustrates the applicability and impact of mechanistic absorption modelling to support oral formulation development and improve the prediction of drug bioavailability from immediate-release formulations.

GastroPlus™ 9.5 Release Webinar: Something for Everyone
We are pleased to announce the release of GastroPlus™ 9.5! This version has something for all users of our top-ranked PBPK modeling platform.

ADMET Predictor 8.1: Efficiently handle LARGE data sets
Building off of the successful launch of the redesigned ADMET Predictor 8.0, many substantial enhancements have been made throughout version 8.1 to improve performance.

Incorporating transporter kinetics into PBPK models
This webinar will focus more on transporters involved in drug distribution and elimination. A brief summary of current information on transporter expression levels will be followed by a description of options for including transporters in the GastroPlus™ PBPK model.

GastroPlus™ PBPK modeling as a tool to ensure target performance of a generic drug product
For a generic drug, the objective is to develop a formulation/process that would result in the product performance equivalent to that of the reference (innovative) product throughout the product life-cycle.

PKPlus™ – The next-generation software for preclinical and clinical trial data analysis
Every lead compound that enters preclinical testing warrants some form of noncompartmental analysis (NCA), with promising candidates that are heading into clinical trials requiring validated NCA as part...

Using GastroPlus™ Modeling to Eliminate Bridging Clinical Studies for Late
This webinar will focus on 2 case studies from scientists from Roche and Janssen describing the use of GastroPlus PBPK modeling to eliminate bridging clinical studies.

ADMET Predictor – Your user experience redefined…
The user interface in ADMET Predictor was completely rewritten for version 8. In this webinar, we demonstrate the new tools available in ADMET Predictor 8 using examples from various data sets, e.g., BACE1 inhibitors.
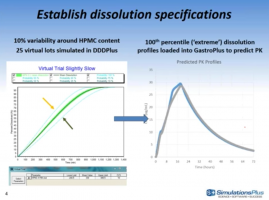
Reimagine the In Vitro Dissolution Experiment with DDDPlus™ 5.0…
In this webinar, case studies will be presented highlighting the use of DDDPlus and GastroPlus to optimize formulations and apply virtual 'lot-to-lot' variability effects to help establish dissolution specifications.

Unified dissolution / precipitation model and its use predicting absorption
This webinar will focus on the description of a novel mechanistic mathematical model developed to describe in vitro dissolution and precipitation data and how the model can be used in a wider PBPK framework...
Application of Cellular Permeability Simulation and PBPK Models to Capture
In this GastroPlus™ User Group webinar, we will discuss the validation of passive permeability estimates in MembranePlus™ based on molecular structure alone for a library of diverse compounds and how...

Applications of customized GastroPlus™ models
This GastroPlus™ User Group video explores how the ACAT model, with customized refinement of the model parameters or design of the model structure, can provide valuable insights to address various aspects of...
Analyzing the structural sensitivity of QSAR models using matched molecular
This video explores analyzing the structural sensitivity of QSAR models using matched molecular pairs using MedChem Studio™

Applications and considerations of drug exposure predictions in pediatric
This GastroPlus™ User Group webinar will focus on special considerations when performing pediatric PBPK modeling in GastroPlus™.
What’s New in ADMET Predictor™ 7.2?
Two new models, fraction unbound to liver microsomes and unbound human liver microsomal intrinsic clearance, were added to ADMET Predictor 7.2.
What’s New In GastroPlus™ 9.0?
This informative webinar covers the new features in the latest version of GastroPlus™ 9.0.
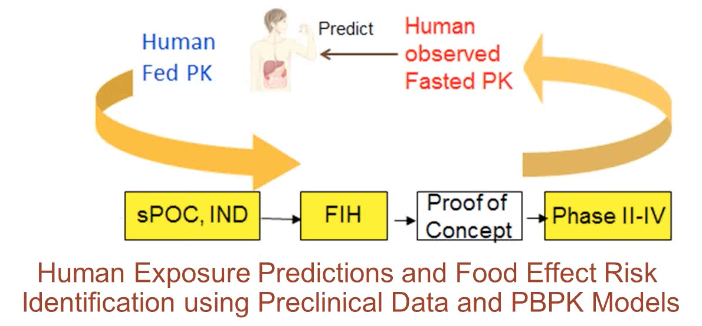
Human Exposure Predictions and Food Effect Risk Identification
This GastroPlus™ User Group webinar explores human exposure predictions and food effect risk identification using preclinical data and PBPK models.

Application of PBPK Modeling in Generic Drug Evaluation
This GastroPlus™ User Group/FDA webinar provides a few examples of modeling and simulation in the OGD for the purpose of addressing biopharmaceutical performance questions for orally administered generic drug products. View slides from this webinar.
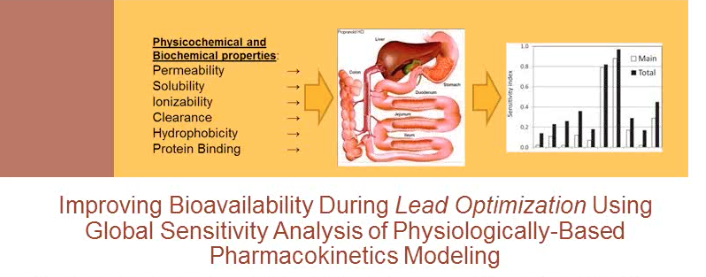
Improving Bioavailability During Lead Optimization
In this presentation, Dr. Eric Martin of Novartis discusses improving bioavailability during lead optimization using global sensitivity analysis (GSA) of physiologically based pharmacokinetics.
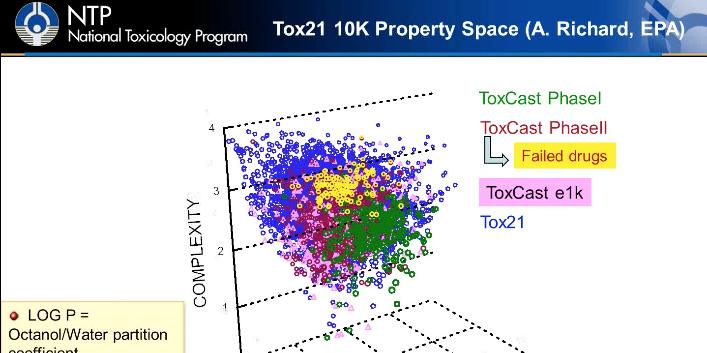
Analysis of the Tox21 10k Library with In Silico QSAR Models for Xenobiotic Metabolism and Toxicity
In this webinar Dr. Stephen Ferguson of the National Institutes of Environmental Health Sciences discusses in silico approaches to predict human xenobiotic metabolism and their potential for human toxicity.

