DILIsym Services, Inc. (DSSI) is a Simulations Plus company...

QST Applications, Use of Data and Species Differences
DILIsym Services, Inc. (DSSI) is a Simulations Plus company..

DILIsym Simulations Support the Liver Safety of Ubrogepant in New Toxicological Sciences Publication
Dr. Paul B. Watkins and Dr. Jeff Woodhead discuss the DILIsym analysis of the latest publication in Toxicological Sciences! Small-molecule calcitonin gene...

Modeling DILI Drug-Drug Interactions with DILIsym
DILIsym Services, Inc. offers comprehensive program services:

Physiologically Based Biopharmaceutics Modeling and Virtual Bioequivalence Assessment to Support Formulation Development
Mechanistic Absorption and PK modeling using GastroPlus®

Annual Shareholders’ Meeting
Our mission is to improve the productivity of science-based research & development enterprises by delivering innovative modeling and simulation software and insightful consulting services

Simulations Plus to Present at the 22nd Annual Needham Growth Conference
Simulations Plus to Present at the 22nd Annual Needham Growth Conference

LD Micro 12th Annual Main Event Investor Conference
Our mission is to improve the productivity of science-based research & development enterprises by delivering innovative modeling and simulation software and insightful consulting services

QSP/QST Modeling Support for NASH Drug Development
Quantitative Systems Pharmacology (QSP) Supports Clinical Development by Emphasizing Mechanistic Understanding of Pathophysiology and Treatment

Benchmark 8th Annual Discovery Conference
Our mission is to improve the productivity of science-based research & development enterprises by delivering innovative modeling and simulation software and insightful consulting services

Mechanistic Insights into Drug-Induced Liver Injury for Macrolide Antibiotics using Quantitative Systems Toxicology Modeling
DILIsym Services Is Using QSP and QST Modeling to Predict Efficacy and Safety of Drugs in Development
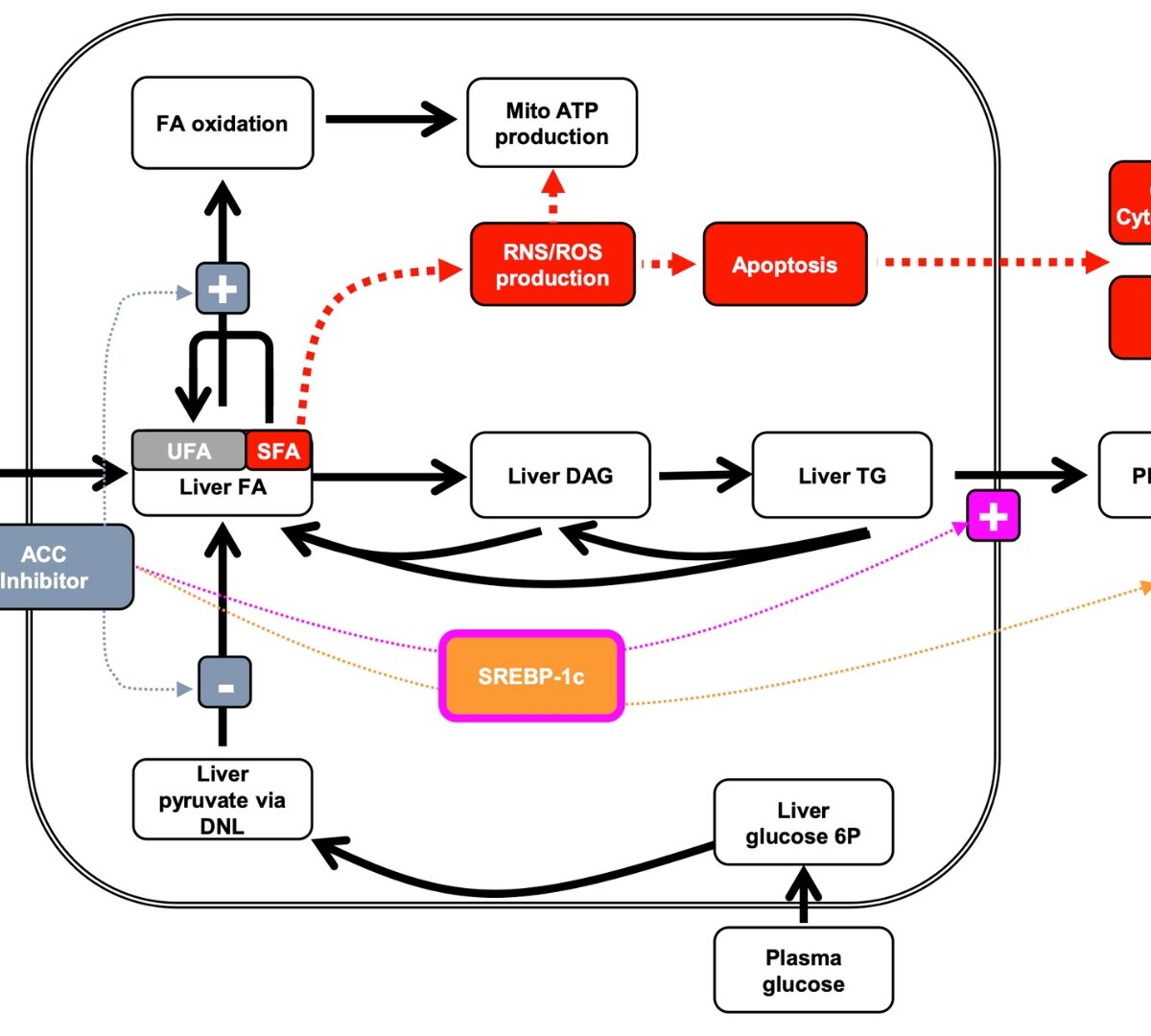
Development of Drugs to Treat NAFLD/NASH using Quantitative Systems Pharmacology Modeling
This session will provide scientific background and overview of the application of quantitative systems pharmacology (QSP) modeling in drug development to treat NALFD/NASH

Different Perspectives – Informing Drug Development Decision Making Through Complementary M&S Approaches
Although fundamental principles are different between PBPK and population PK approaches, both techniques have been used to study drug disposition during drug development.

Mechanistic PBPK modeling of special population groups – considerations and opportunities
Unwarranted studies, due to the general nature of regulatory guidelines, may be avoided.

Utilizing DILIsym, a QST Platform, to Extract More from Your Data to Support Decisions
Recently Completed Clinical Trial Indicates that DILIsym Backup Compound Predictions Were Correct

New approach to regression uncertainty analysis and applications to drug design
When can you trust a decision/prediction from a machine learning model?

Using Quantitative Systems Toxicology (QST): Improving the Safety of Drugs While Reducing Animal Testing
QST modeling is having a significant impact on decision making in drug development.

How to obtain biowaivers for clinical trials using PBPK models, two case studies
FDA is open to proposals of using modeling approaches for bioequivalence (BE), or for new drugs,with the proper justification and model verification

Advancing current PBPK model applications to support internal development and regulatory decisions
Numbers of applications (NDA, ANDA) supported by PBPK modeling has increased significantly since 2008.

In Silico and in Vitro Simulations to Predict Idiosyncratic DILI: What is on the Horizon?
Multiple species: human, rat, mouse, and dog, Population variability, The three primary acinar zones of liver represented, Essential cellular processes represented to multiple scales in interacting submodels...
