Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI

Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI
Integrating Human Biomimetic Liver Microphysiology System with Quantitative Systems Toxicology Modeling to Predict DILI

Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)
ASCPT 2024: Construction of a Simulated Population of Post-Menopausal Women for the Prediction of Drug-Induced Liver Injury (DILI)

Lunch & Learn – SOT 63rd Annual Meeting and ToxExpo
SOT 2024: What's New at Simulations Plus, Your Partner in Winning, for Exposure and Safety Assessment

Simulations Plus Investor Day 2023
Putting Clients First Drives Growth and Fuels Innovation

Contributed data, collaboration and experience with ionization models – Part 1. Bayer Pharmaceuticals
World‘s best in class pKa prediction tool

Utilizing pKa Values for Effective Crop Protection: On Systemicity and Efficacy of Molecules
pKa important factor in research screening cascade of molecules

Improving pKa modelling through active collaborations
What needs to be completed for collaboration?

Impact of collaborative pKa modelling
Importance of pKa in Drug Discovery

The application of AI-driven Drug Discovery technology for molecular optimization of nuclear receptor ligands
ADMET Predictor® as a de novo drug design environment

Applying Mechanistic PBPK Modeling and Simulations to Support Regulatory Interactions
Physiologically-Based Pharmacokinetic (PBPK) Modeling is a Tool/Component of Model-Informed Drug Development (MIDD)

Model conception, parameterization using in silicomethods, and computational implementation
The PKB model structure depends on the problem, knowledge of the biokinetic mechanism, and availability of suitable data

Application of Modeling and Simulation in Long-Acting Injectable Product Development
Discuss advances in mechanistic models to simulate in vitro and in vivobehavior of long acting injectables.

From Preclinical to Clinical Drug Product Development: A Path for Smooth Transition
It is challenging to establish a standard approach for moving from preclinical to clinical phases of development. Many hurdles exist during formulation selection and for extrapolation of doses from animals to humans; what may have worked well for one drug candidate may not be appropriate for others. This presentation will provide a general framework/strategy to follow when moving from preclinical to clinical studies (FIH) based on risk identification and mitigation focusing on the application of mechanistic modeling and simulation (PBPK).

Absorption Predictions: Current Capabilities and Knowing the Gaps
Number of different processes contribute to net drug absorption

Complex Delivery Routes and Generics: The Next Frontier for PBBM/PBPK Modeling
Generic drug development for locally acting drug products is a fast-growing field of interest

Data required to build a fit-for-purpose PBPK model: How much is necessary?
PBPK/PBBM model development can be initiated at any stage of product development
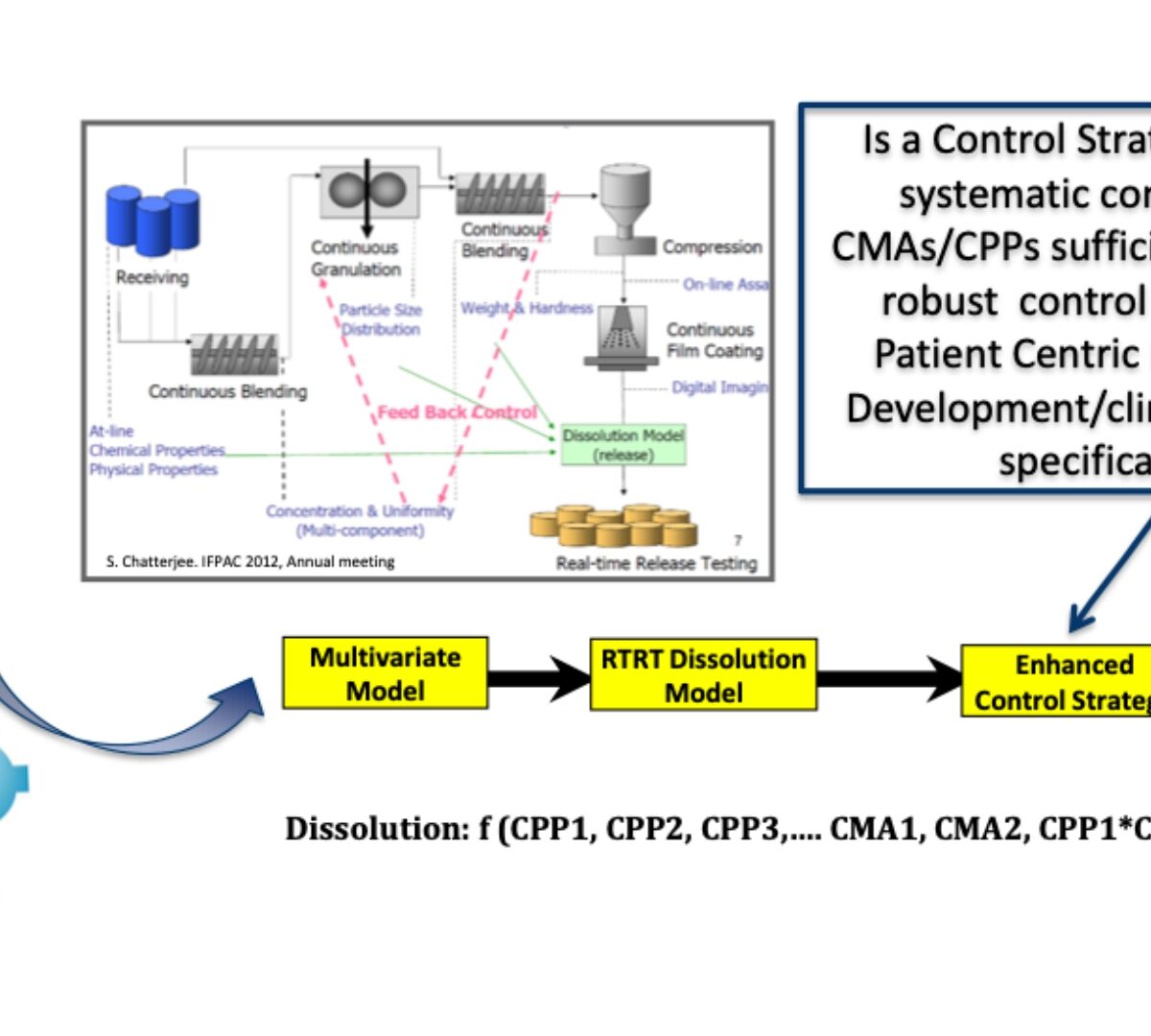
Predictive Dissolution Modeling with Clinically Relevant Specifications
The role of biopharmaceutics risk assessment

Simulations Plus General Overview
Our solutions inform the entire drug development process
