Postbariatric altered gastrointestinal (GI) anatomy/physiology may significantly harm oral drug absorption and overall bioavailability.

DNDI-6174 is a preclinical candidate for visceral leishmaniasis that targets the cytochrome bc
New drugs for visceral leishmaniasis that are safe, low cost, and adapted to the field are urgently required.

Evaluating the Role of N-Acetyl-L-Tryptophan in the Aβ 1-42-Induced Neuroinflammation and Cognitive Decline in Alzheimer’s Disease
Alzheimer’s disease (https://www.simulations-plus.com/wp-admin/post.php?post=35654&action=editAD), a neurodegenerative condition previously known to affect the older population, is also now seen in younger individuals.

Physiologically Based Pharmacokinetic Modeling in Neonates: Current Status and Future Perspectives
Rational drug use in special populations is a clinical problem that doctors and pharma-cists must consider seriously.

Log D7.4 and plasma protein binding of synthetic cannabinoid receptor agonists and a comparison of experimental and predicted lipophilicity
The emergence of new synthetic cannabinoid receptor agonists (SCRAs) onto the illicit drugs market continues to cause harm, and the overall availability of physicochemical and pharmacokinetic data for new psychoactive substances is lacking.

Role of Physiologically Based Biopharmaceutics Modeling (PBBM) in Fed Bioequivalence Study Waivers: Regulatory Outlook, Case Studies and Future Perspectives
Over the past few decades, physiologically based biopharmaceutics modeling (PBBM) has demonstrated its utility in both new drug and generic product development.

In silico prediction of bioequivalence of atorvastatin tablets based on GastroPlus™ software
The prediction of intestinal absorption of various drugs based on computer simulations has been a reality.

Cross species extrapolation of the disruption of thyroid hormone synthesis by oxyfluorfen using in vitro data, physiologically based pharmacokinetic (PBPK), and thyroid hormone kinetics models
The thyroid hormones play key roles in physiological processes such as regulation of the metabolic and cardiac systems as well as the development of the brain and surrounding sympathetic nervous system.
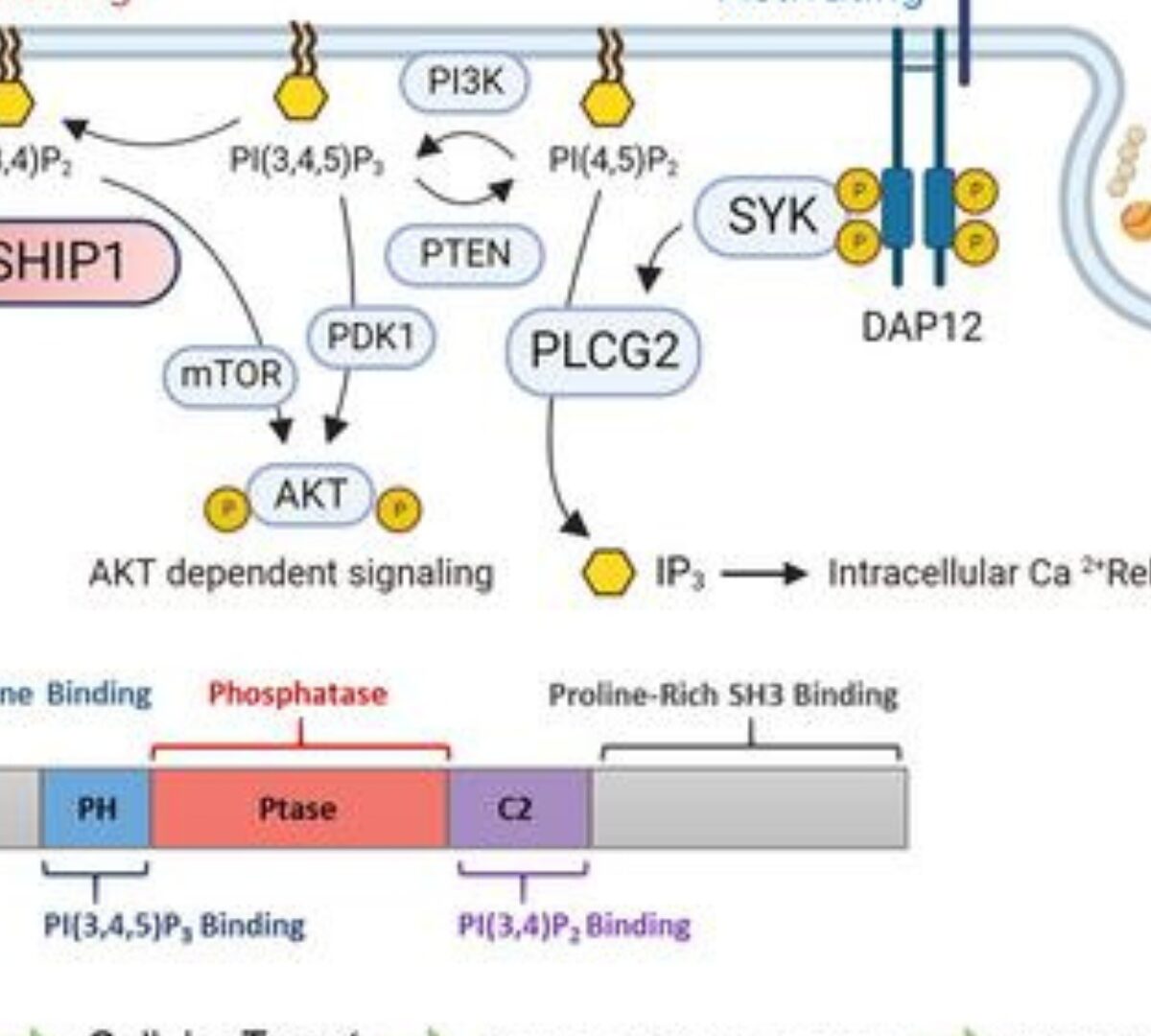
SHIP1 therapeutic target enablement: Identification and evaluation of inhibitors for the treatment of late-onset Alzheimer’s disease
The risk of developing Alzheimer's disease is associated with genes involved in microglial function.
Predicting the pharmacokinetics and pharmacodynamics of antisense oligonucleotides: an overview of various approaches and opportunities for PBPK/PD modelling
Advances in research and development (R&D) have enabled many approvals of antisense oligonucleotides (ASOs). Its administration expanded from systemic to local for treating various diseases, where predicting target tissue exposures and pharmacokinetics (PK) and pharmacodynamics (PD) in human can be critical.
Physiologically Based Pharmacokinetic modelling of drugs in pregnancy: A mini-review on availability and limitations
Physiologically based pharmacokinetic (PBPK) modelling in pregnancy is a relatively new approach that is increasingly being used to assess drug systemic exposure in pregnant women to potentially inform dosing adjustments.
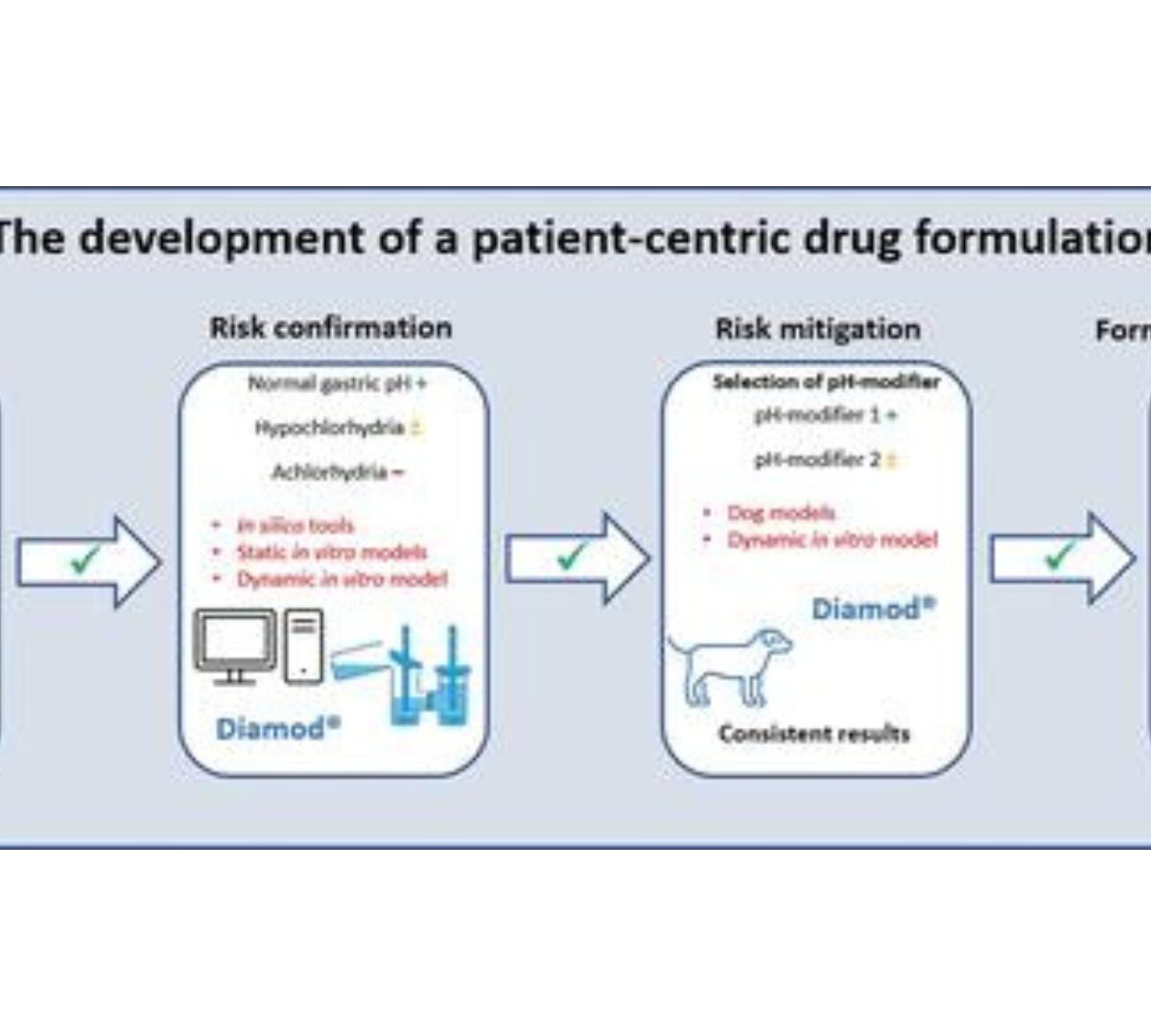
Contribution of the Dynamic Intestinal Absorption Model (Diamod) to the Development of a Patient-Centric Drug Formulation
Compound X is a weak basic drug targeting the early stages of Parkinson’s disease, for which a theoretical risk assessment has indicated that elevated gastric pH conditions could potentially result in reduced plasma concentrations.
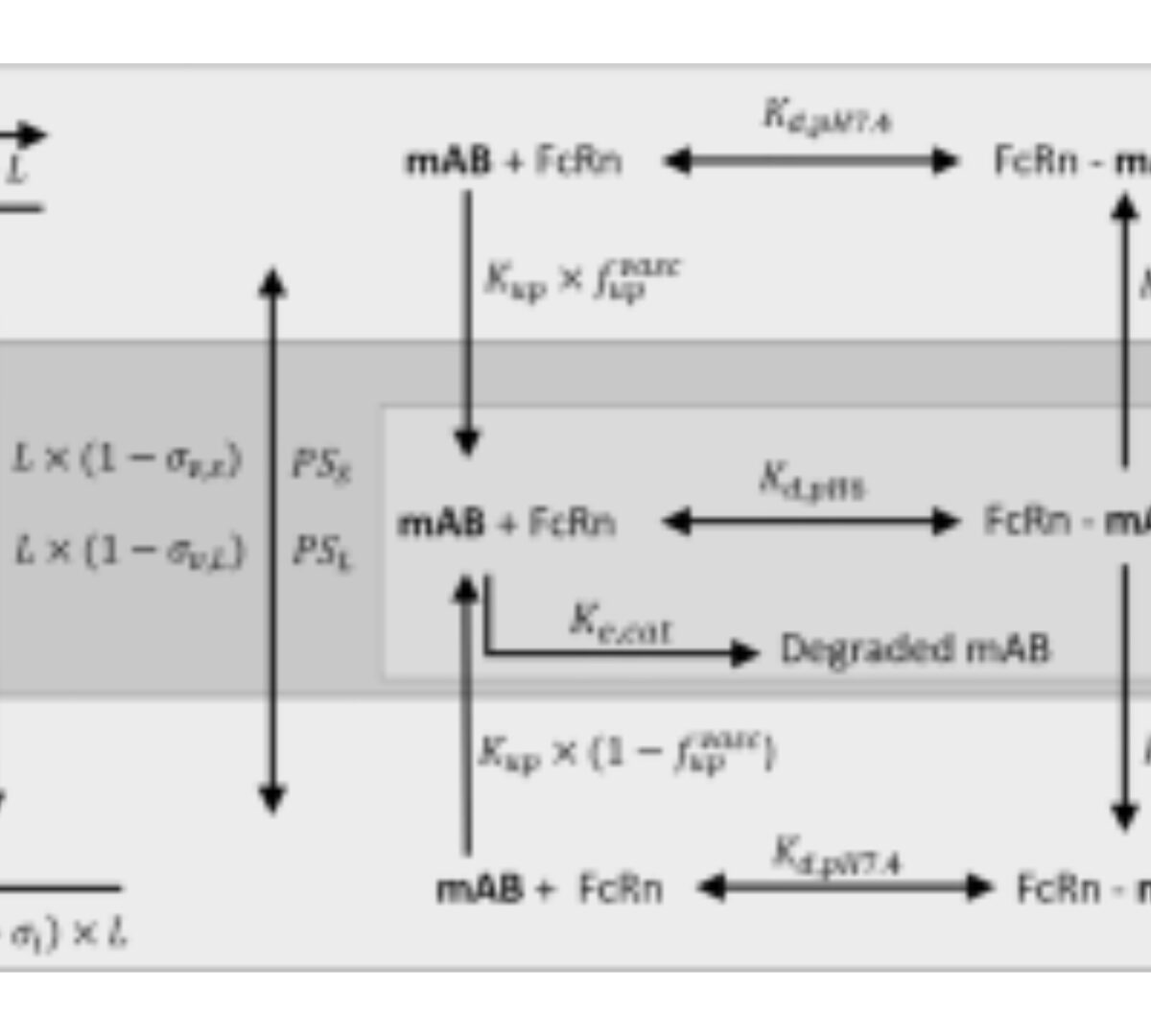
Comparison of monoclonal antibody disposition predictions using different physiologically based pharmacokinetic modelling platforms
Physiologically based pharmacokinetic (PBPK) models can be used to leverage physiological and in vitro data to predict monoclonal antibody (mAb) concentrations in serum and tissues.

Comparison of monoclonal antibody disposition predictions using different physiologically based pharmacokinetic modelling platforms
Physiologically based pharmacokinetic (PBPK) models can be used to leverage physiological and in vitro data to predict monoclonal antibody (mAb) concentrations in serum and tissues.
Use of In silico Methodologies to Predict the Bioavailability of Oral Suspensions: A Regulatory Approach
Oral suspensions are heterogeneous disperse systems, and the particle size distribution, crystalline form of the dispersed solid, and composition of the formulation can be listed as parameters that control the drug dissolution rate and its bioavailability.
Voices in Molecular Pharmaceutics: Meet Dr. Bart Hens, A Sociable Scientist Focusing on Multidisciplinary Connections to Unravel the Gaps of Oral Drug Behavior in the Human Gastrointestinal Tract
Bart Hens (Pharm.D., Ph.D.) holds a Ph.D. degree in Pharmaceutical Sciences obtained from KU Leuven (Supervisor: Prof. Dr. Patrick Augustijns - Leuven, Belgium).
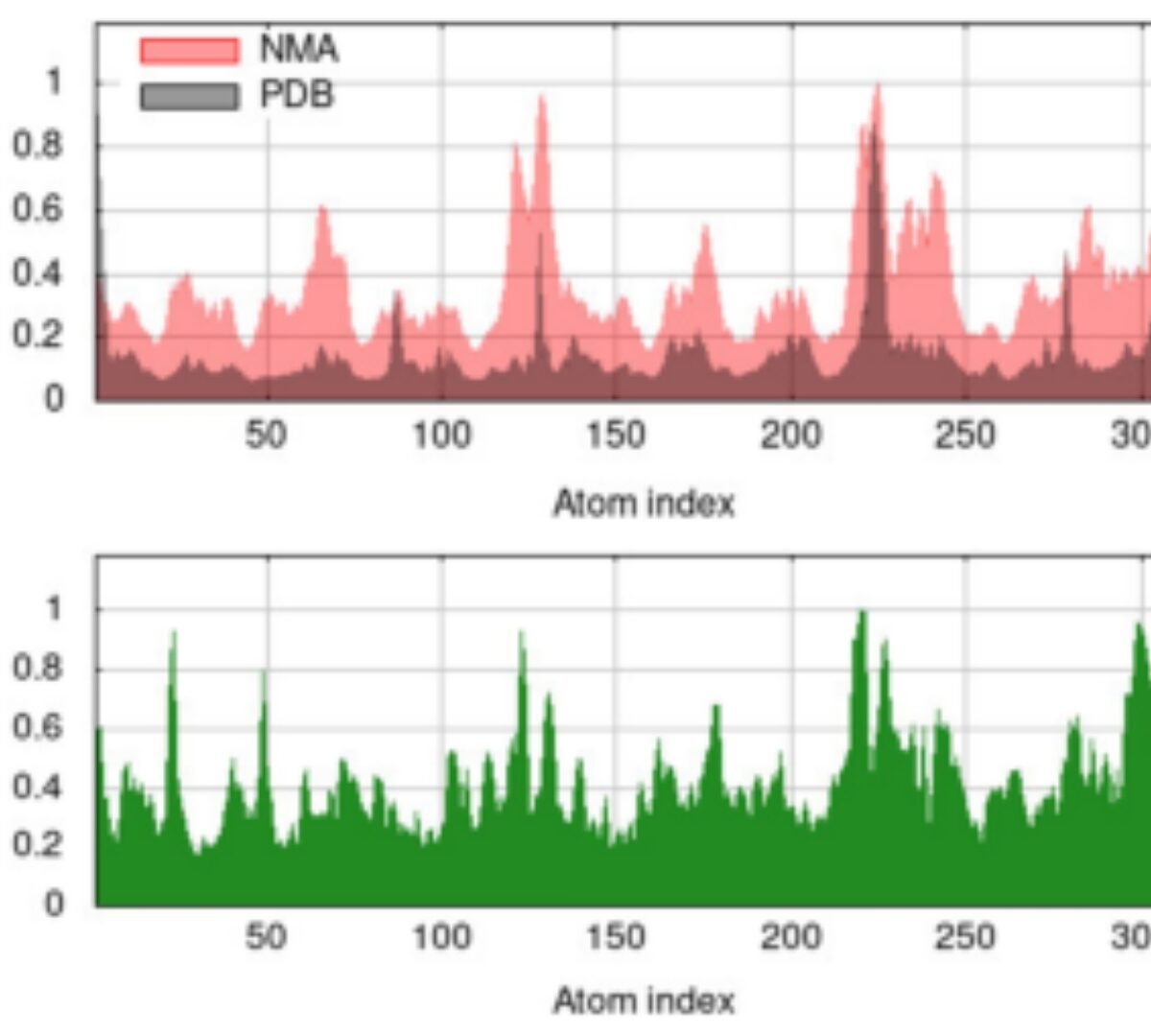
Repurposing of Strychnine as the Potential Inhibitors of Aldo–keto Reductase Family 1 Members B1 and B10: Computational Modeling and Pharmacokinetic Analysis
AKR1B1 and AKR1B10 are important members of aldo–keto reductase family which plays a significant role in cancer progression by modulating cellular metabolism.
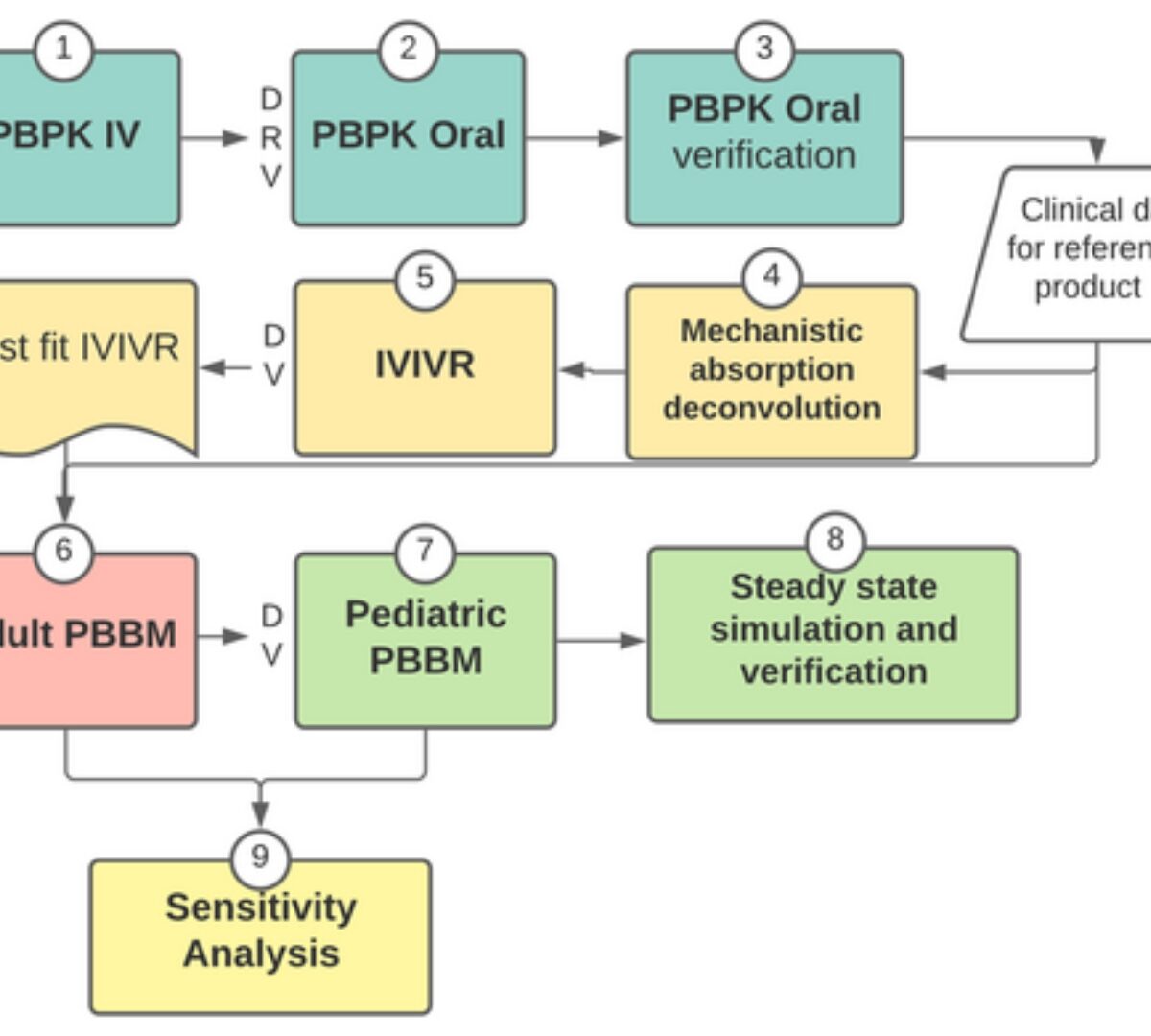
Adult and pediatric physiologically-based biopharmaceutics modeling to explain lamotrigine immediate release absorption process
Physiologically-based biopharmaceutics modeling (PBBM) has potential to accelerate the development of new drug and formulations.
Quercetin-encapsulated magnetoliposomes: Fabrication, optimization, characterization, and antioxidant studies
Quercetin (QU) faces challenges in its therapeutic efficacy due to its hydrophobic nature and limited oral bioavailability.
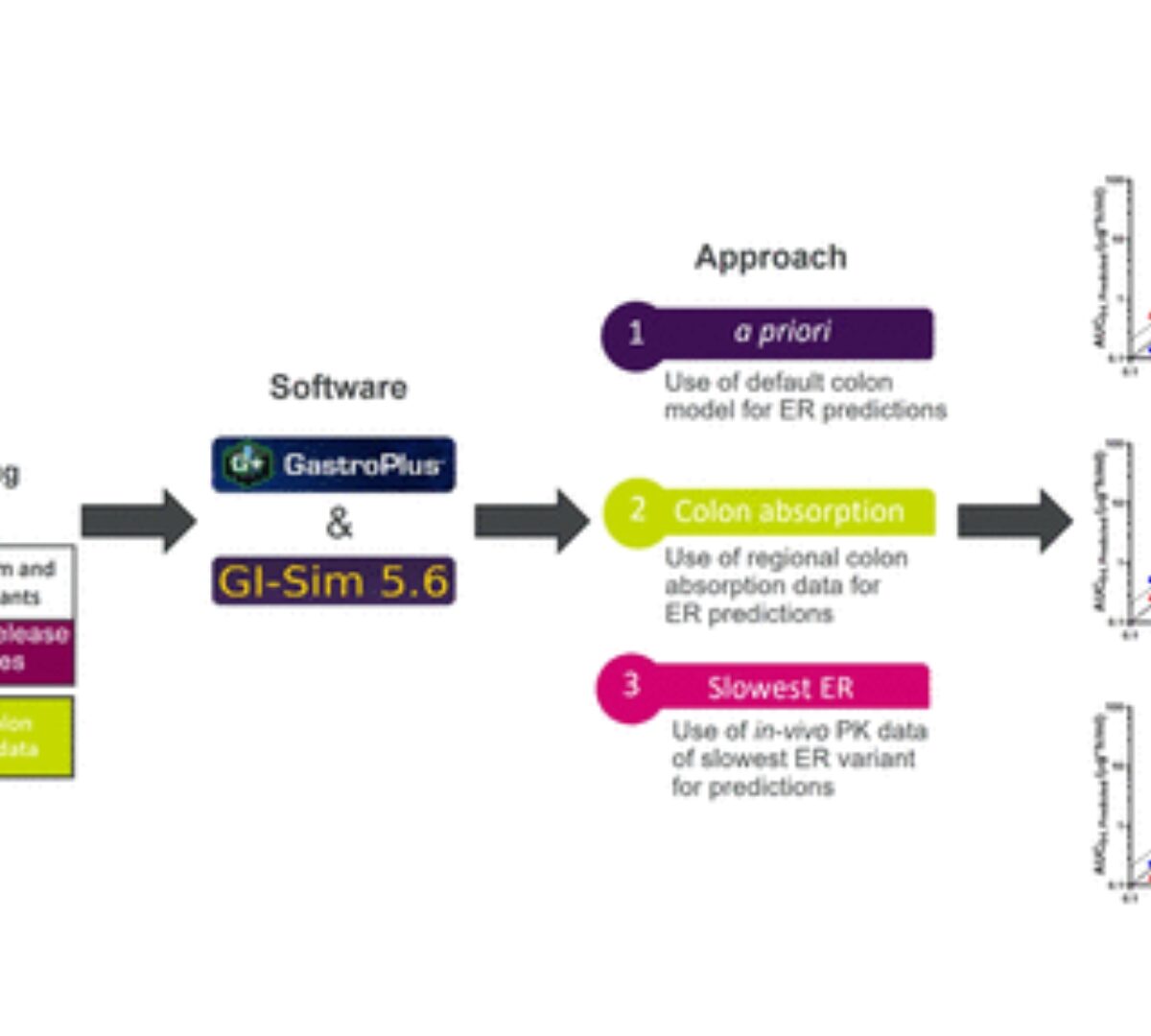
Approaches to Account for Colon Absorption in Physiologically Based Biopharmaceutics Modeling of Extended-Release Drug Products
The rate and extent of colon absorption are important determinants of the in vivo performance of extended-release (ER) drug products.
