To support regulatory decision-making without animal testing, Next-Generation Risk Assessment (NGRA) frameworks leverage New Approach Methodologies (NAM).

Applications of PBPK Models to Predict Tissue Residues and Extralabel Withdrawal Times of Drugs in Food Animals: Perspectives from the Food Animal Residue Avoidance Databank (FARAD) Program
Physiologically based pharmacokinetic (PBPK) models are commonly used in human drug discovery and development and human health risk assessment of environmental chemicals.
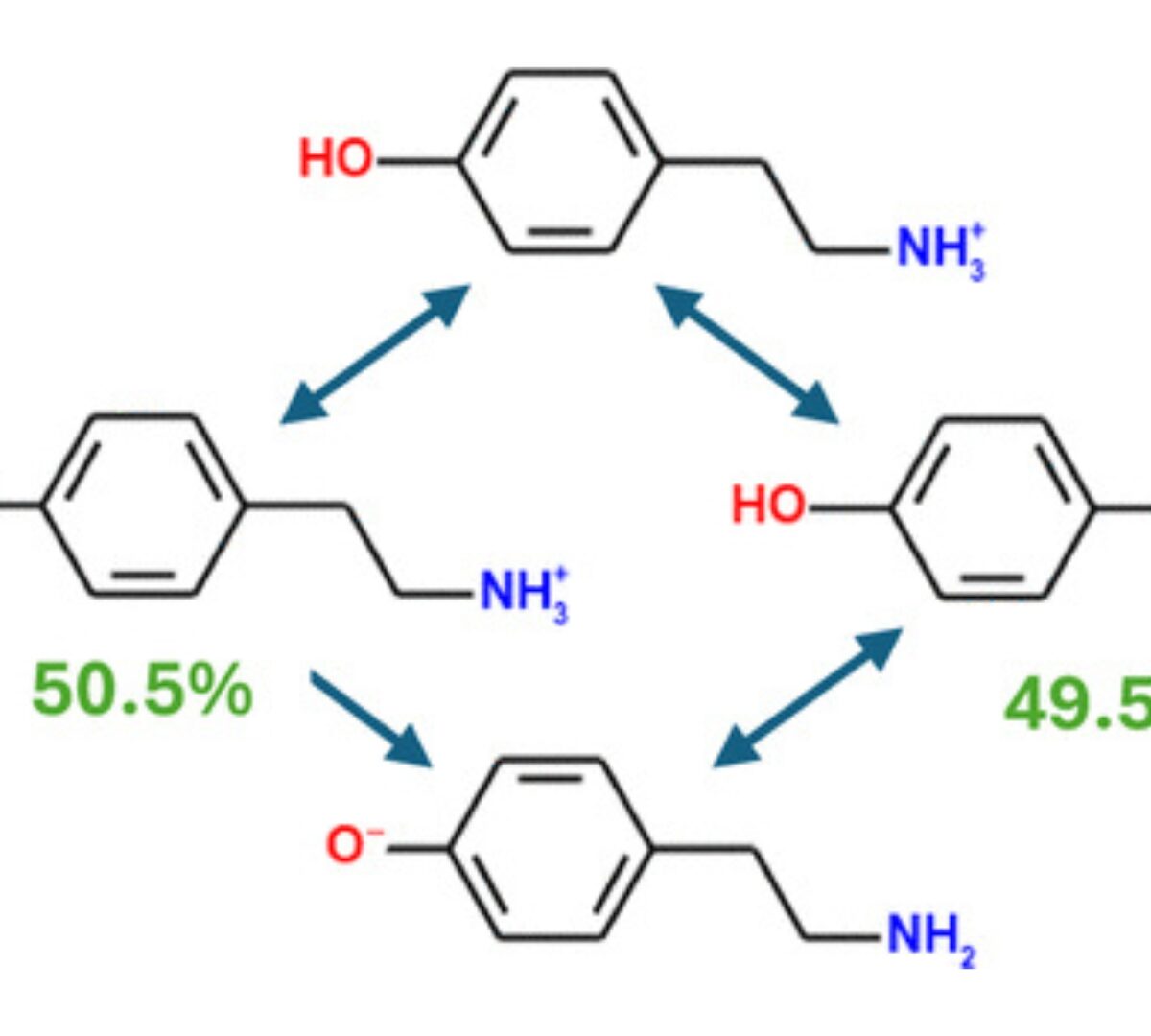
What, This “Base” Is Not a Base? Common Misconceptions about Aqueous Ionization That May Hinder Drug Discovery and Development
The challenges of modern medicinal chemistry increase with the complexity of the chemical compounds studied.
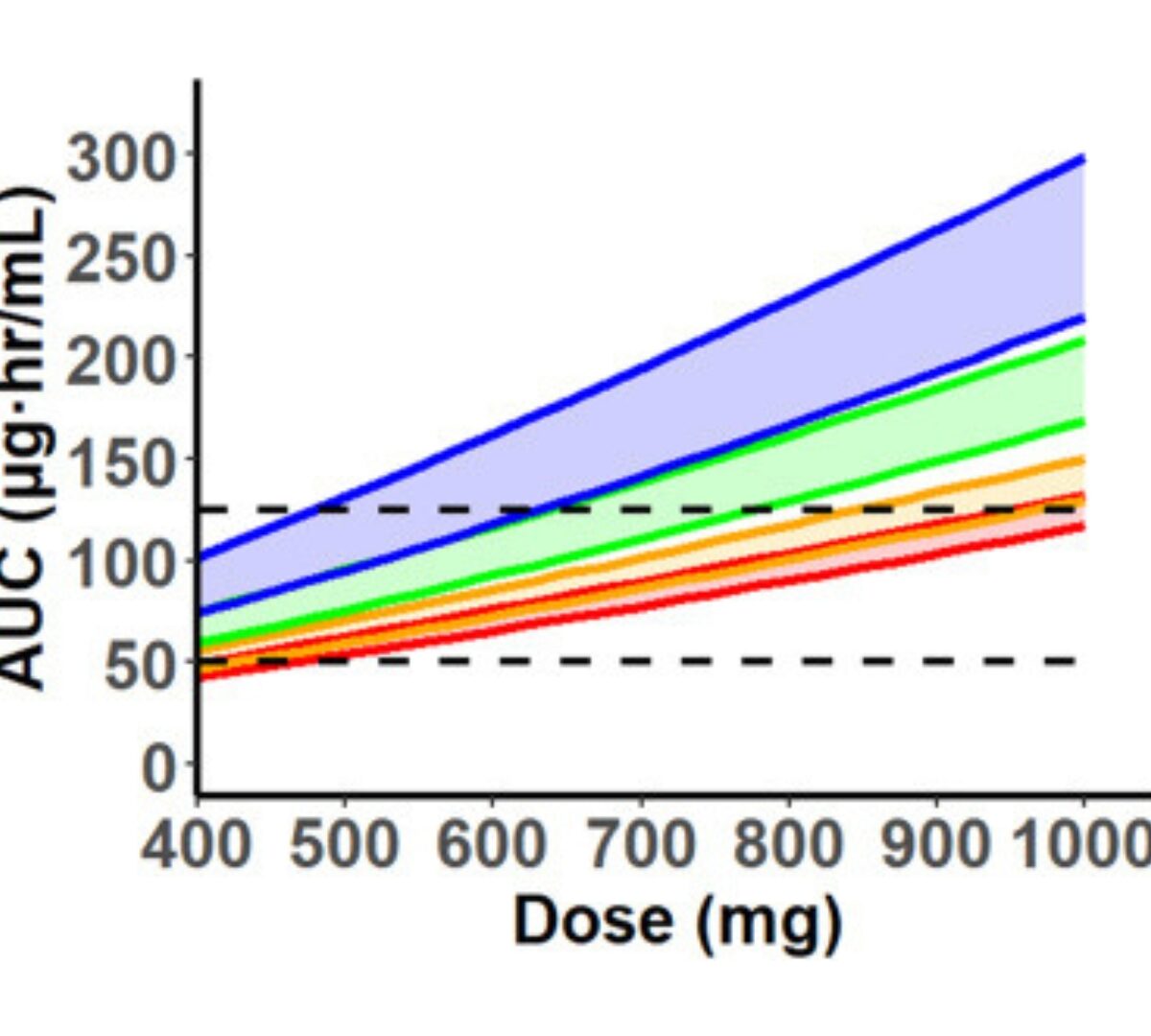
Physiologically Based Pharmacokinetic Modeling of Hydroxyurea: Implications for Dose Adjustment in Patients with Renal Insufficiency
Hydroxyurea is widely used in the management of sickle cell anemia.
Pharmacomicrobiomics
Oral medications encounter gut commensal microbes that participate directly and indirectly in drug effects through metabolism, interactions with drug metabolites, or production of substrates that compete with drugs for drug-metabolizing enzymes, consequently influencing drug pharmacokinetics.
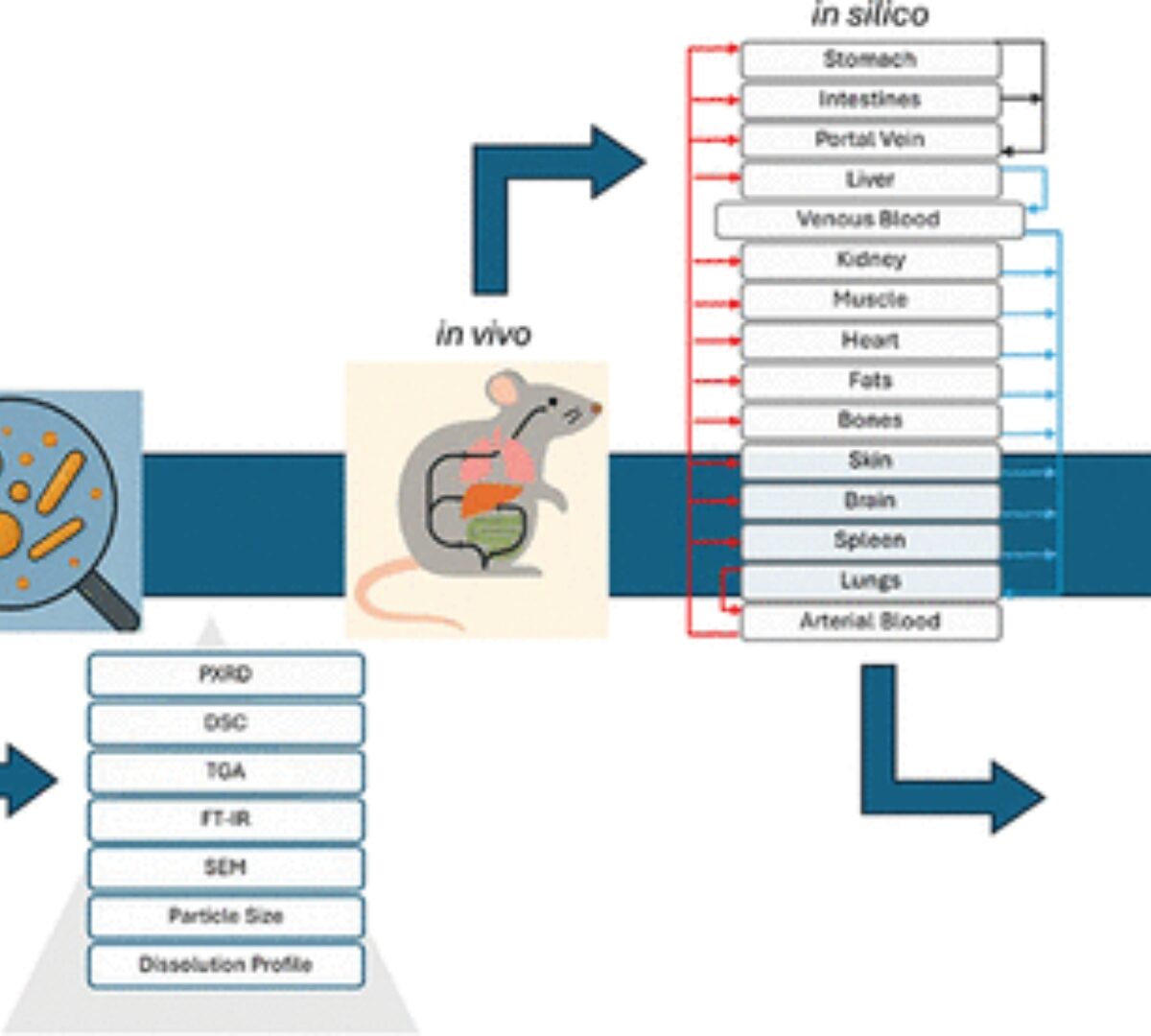
Physiologically Based Pharmacokinetic Modeling of Efavirenz Nanoparticles: from Animal Model to Human Extrapolation
The present work aims to establish a formulation-specific, physiologically based pharmacokinetic (PBPK) model for efavirenz (EFV) nanocrystals that have shown increased dissolution and were produced ...
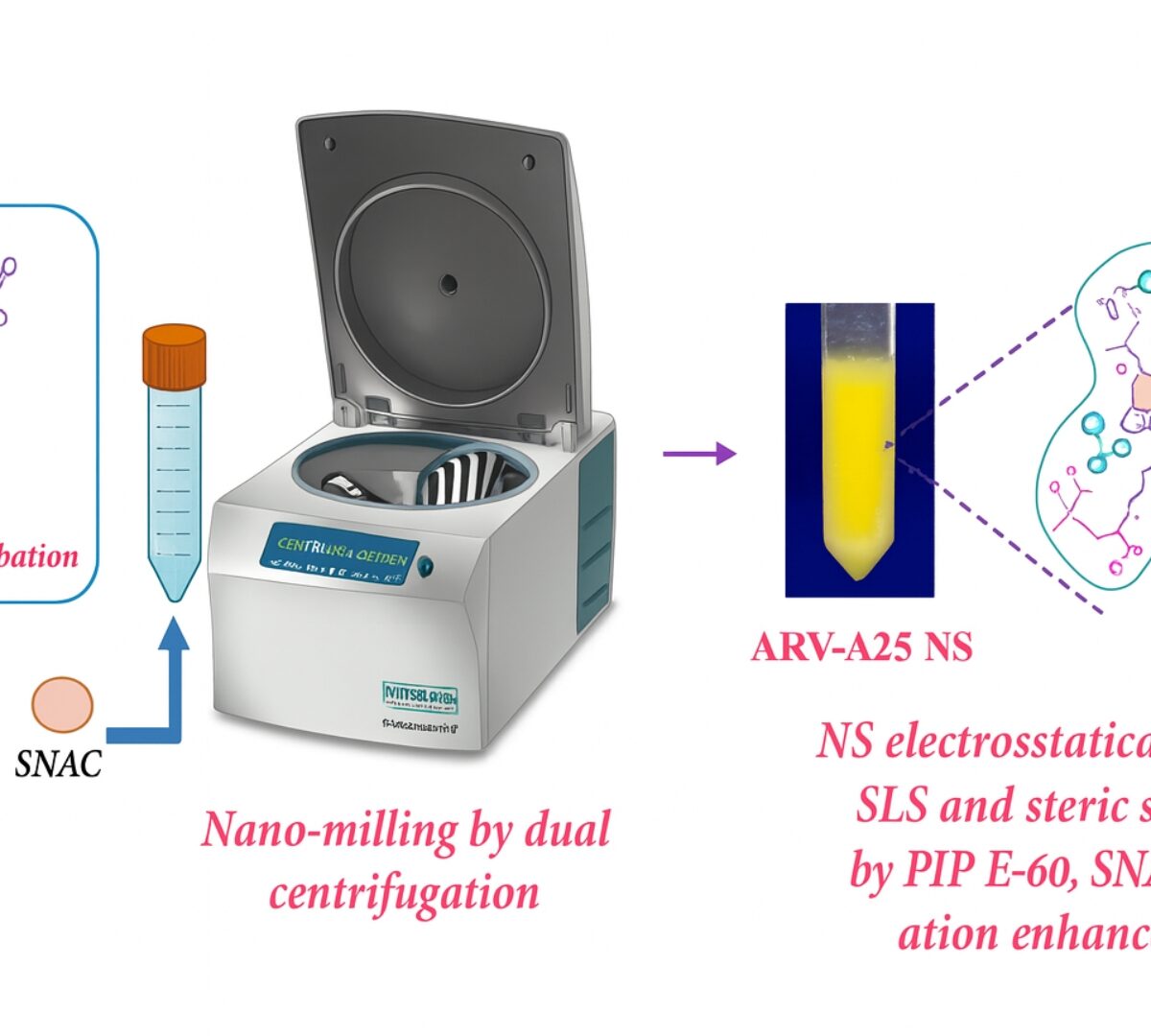
Permeability Enhancer Incorporated Oral Nanosuspension of ARV-825 PROTAC for Glioblastoma Treatment
Glioblastoma(GBM) is an aggressive brain tumor with dismal prognosis, necessitating innovative therapeutic strategies.

Indirect Modeling of Post-Prandial Intestinal Lymphatic Uptake of Halofantrine Using PBPK Approaches: Limitations and Implications
Despite the recognized importance and distinctive characteristics of the intestinal lymphatic pathway in drug absorption, its pharmacokinetic modeling remains largely unexplored.

Innovative Triamcinolone Acetonide Microsuspension for Non-Invasive Ocular Management of Inflammation
Enhancing the bioavailability of insoluble active agents in the eye through topical administration is a key focus in formulation science.

Physiologically Based Pharmacokinetic Modeling and Dose Optimization of Linezolid in Pediatric Patients With Renal Impairment
Linezolid (LZD), a commonly used antimicrobial agent in clinical practice, has not undergone adequate pharmacokinetic (PK) assessment in pediatric populations with renal impairment (RI).

Differential Cannabinoid-Like Effects, Receptor Affinity and Physiologically Based Pharmacokinetics of the Synthetic Cannabinoids 4F-MDMB-BINACA, 4F-MDMB-BICA and 5F-MDMB-PICA in Mice: A Comparative Study
Synthetic cannabinoid receptor agonists (SCRAs) 4F-MDMB-BINACA, 4F-MDMB-BICA, and 5F-MDMB-PICA share a “tail” group but differ in indazole/indole cores and N-fluoroalkyl chain lengths (C4 vs. C5).

In Vivo Pharmacodynamic and Pharmacokinetic Assessment of Cannabidiol-loaded Camel Milk Exosomes in Doxorubicin-Resistant Triple-Negative Breast Cancer Xenografts
Cannabidiol (CBD) suffers from poor aqueous solubility and extensive first-pass metabolism, which significantly limits its oral bioavailability.
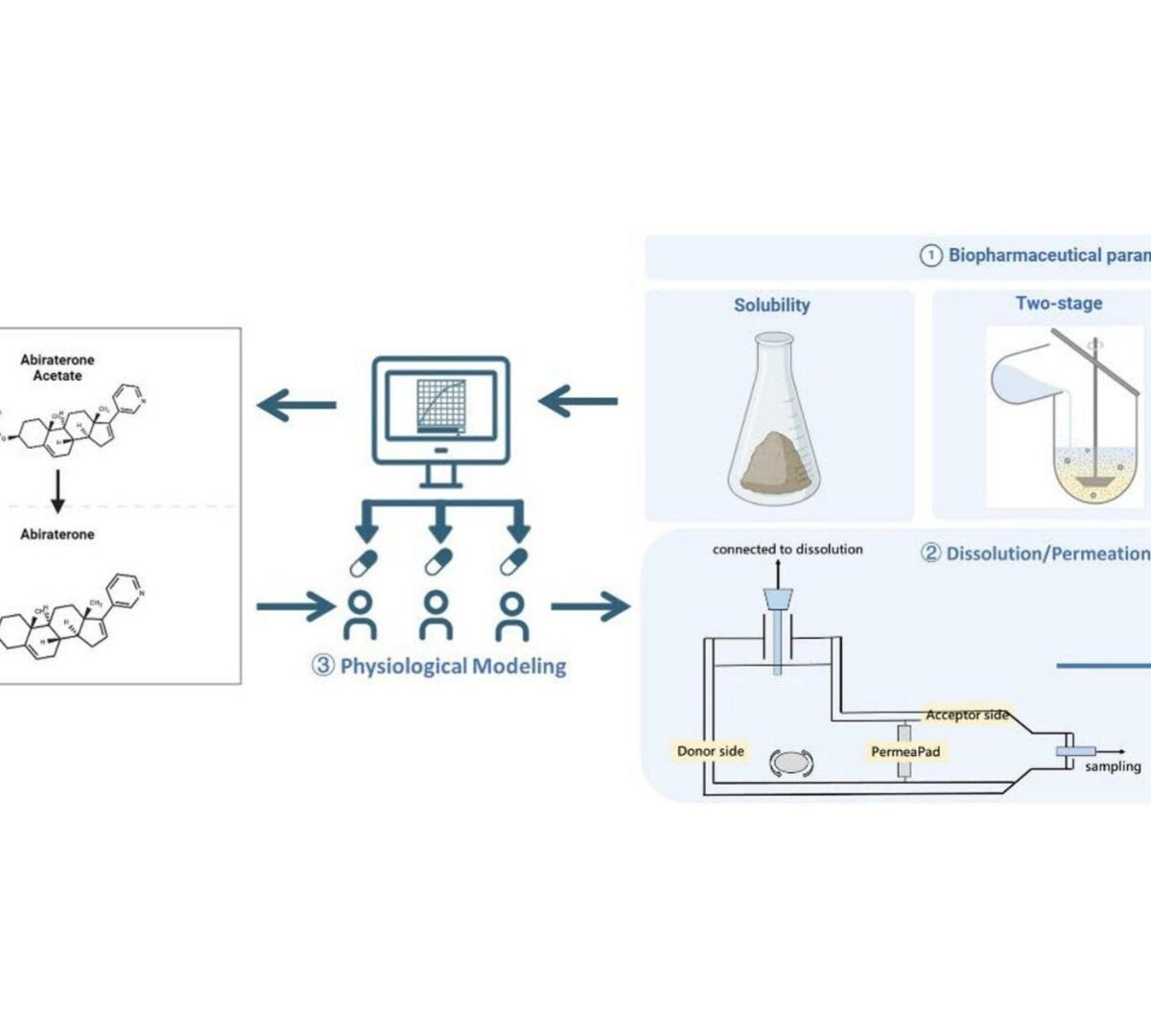
Establishing Clinically Relevant Dissolution Specifications for Prodrug Bioequivalence Risk Assessment: Integration of a Dissolution/Permeation System with Physiologically Based Biopharmaceutics Modeling in Abiraterone Acetate
Prodrugs with enzymatic activation requirements, such as the weakly basic biopharmaceutical classification system (BCS) class IV compound...
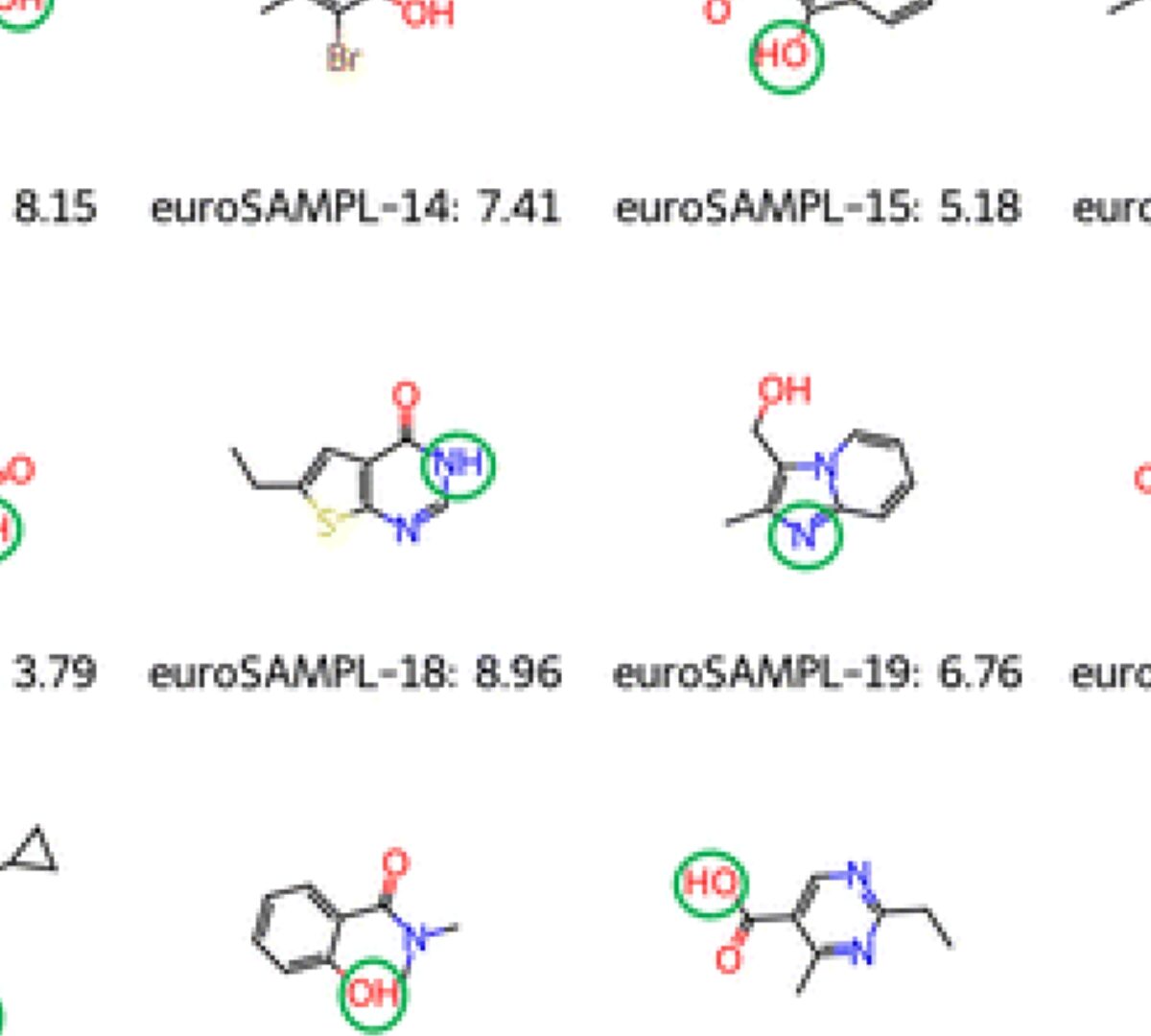
The EuroSAMPL1 pKa Blind Prediction and Reproducible Research Data Management Challenge
The development and testing of methods in computational chemistry for the prediction of physicochemical properties is by now a mature form of scientific research, with a number of different methods ranging from molecular mechanics simulations, over quantum calculations, to empirical and machine learning models.
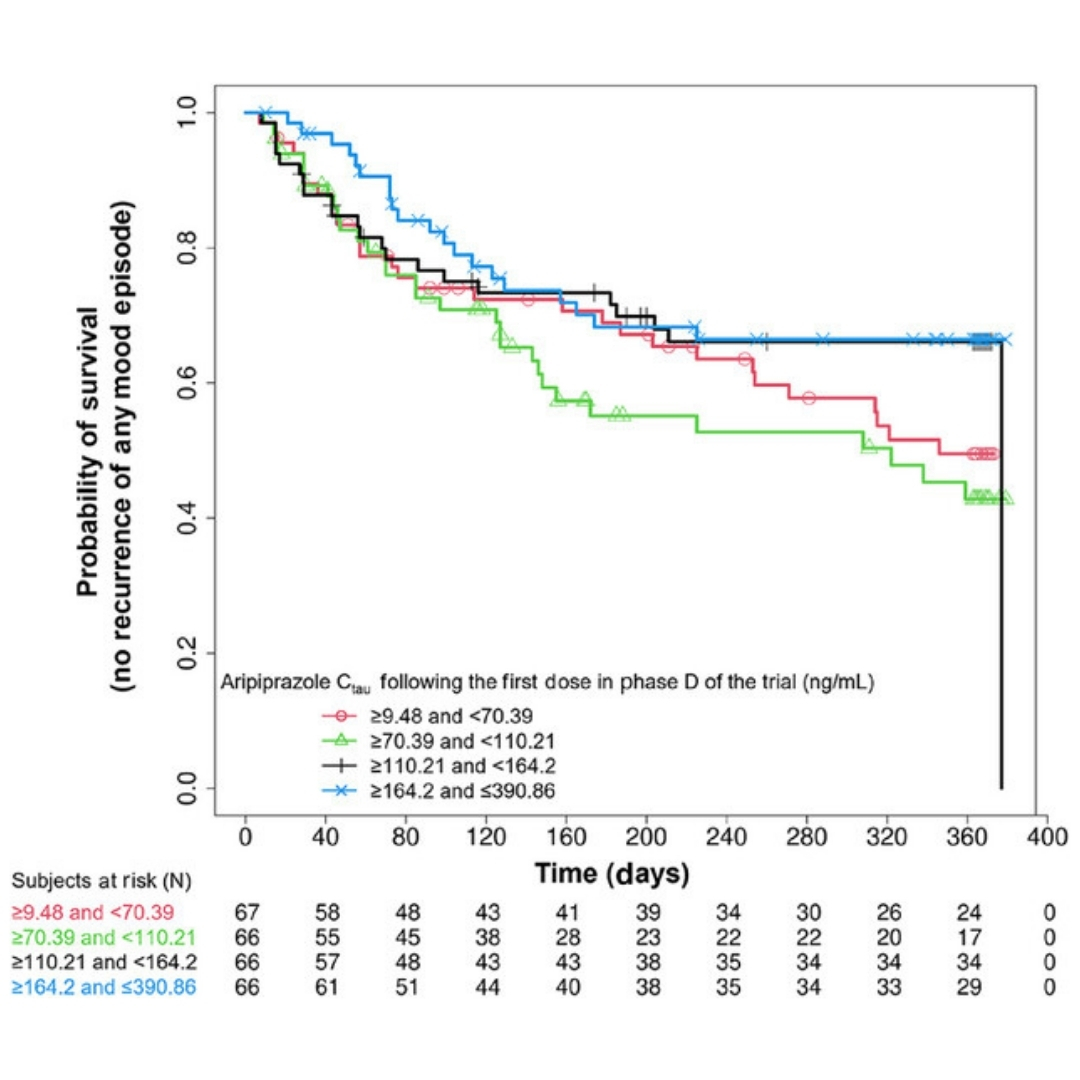
Exposure–Response Analysis for Aripiprazole Once-Monthly in Patients Diagnosed With Bipolar I Disorder
Aripiprazole once-monthly (AOM) is approved for the maintenance monotherapy treatment of bipolar I disorder (BP-I) in adults.
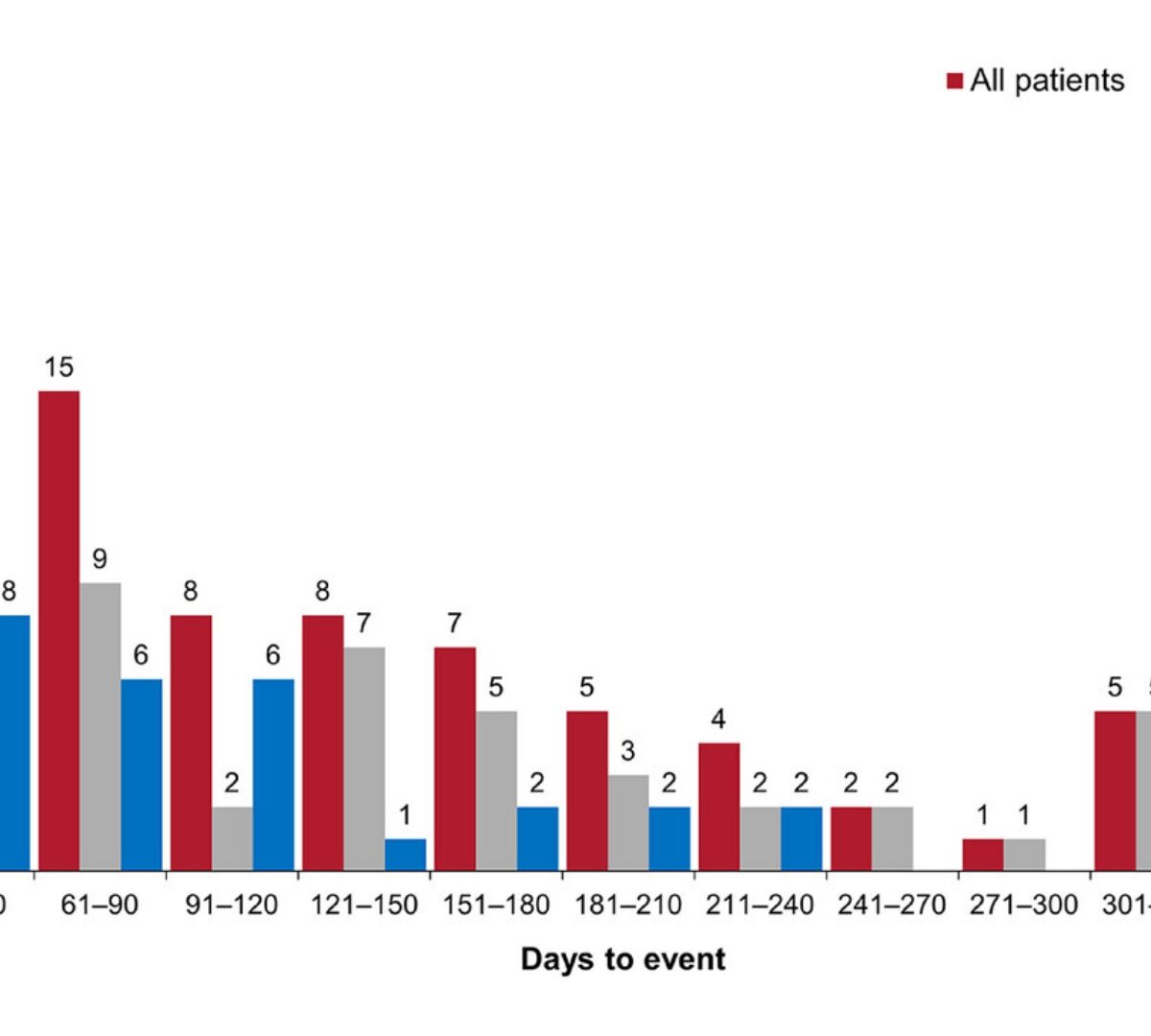
Dissolving Microneedle Patches for Transdermal Delivery of Paroxetine: in-vitro, ex-vivo Studies and its PBPK Modeling
Paroxetine HCl (PRX-HCl), an antidepressant, has poor water solubility and low oral bioavailability with 50% being metabolized in the liver.
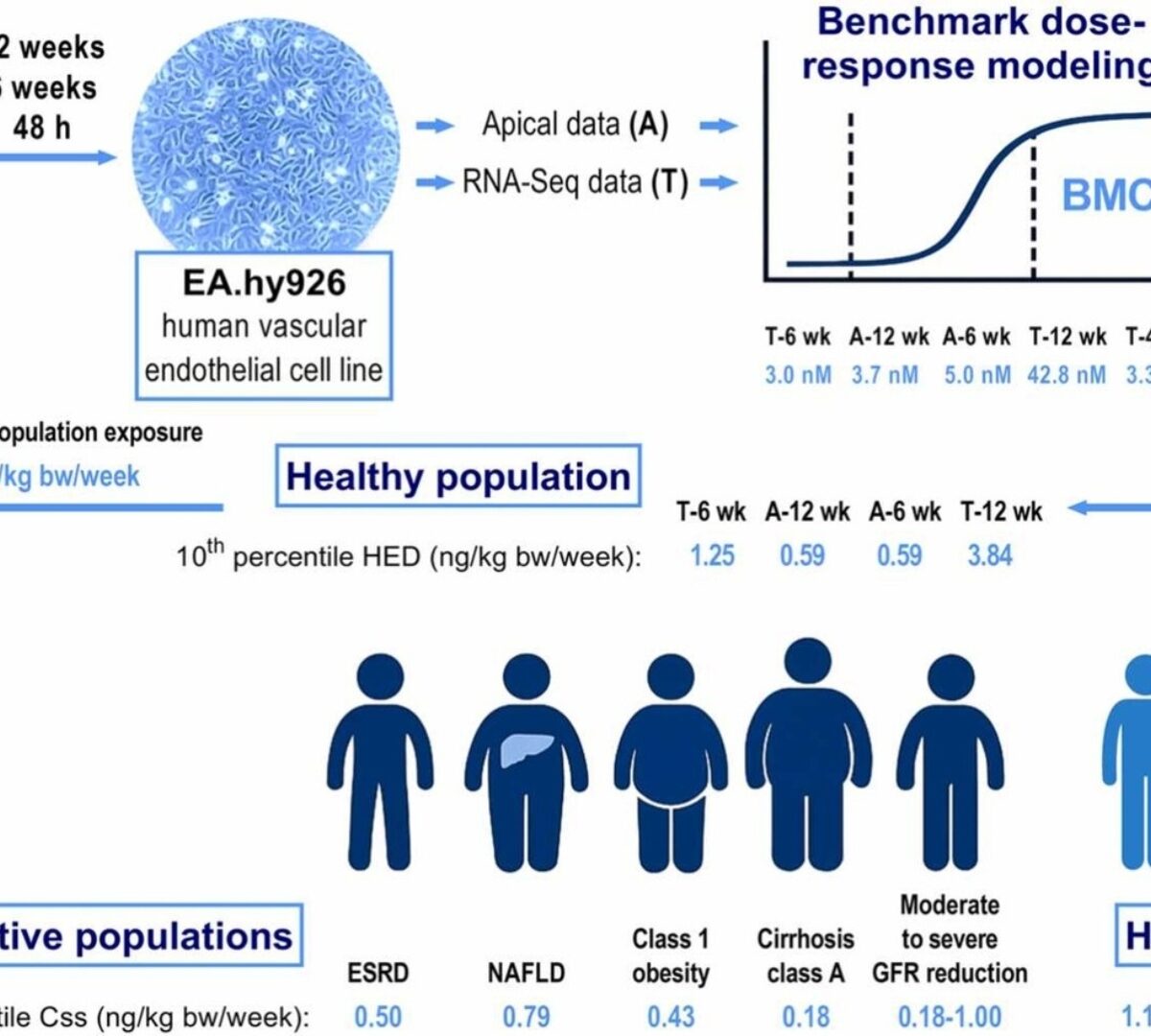
Advancing Probabilistic Risk Assessment of Perfluorooctanoic Acid Through Integration of in vitro Data and Physiologically Based Toxicokinetic Modeling Coupled with Population-Specific Analysis
Current human health risk assessment for perfluorooctanoic acid (PFOA) has proven inadequate due to a lack of innovative approaches.

New Era in Bioequivalence Global Harmonization Through ICH M13 Initiative: Critical Review on Mew Concepts, Alternative Approaches for High-Risk Products
Bioequivalence (BE) studies have made significant advancements, particularly with the introduction of the ICH M13 guidances.
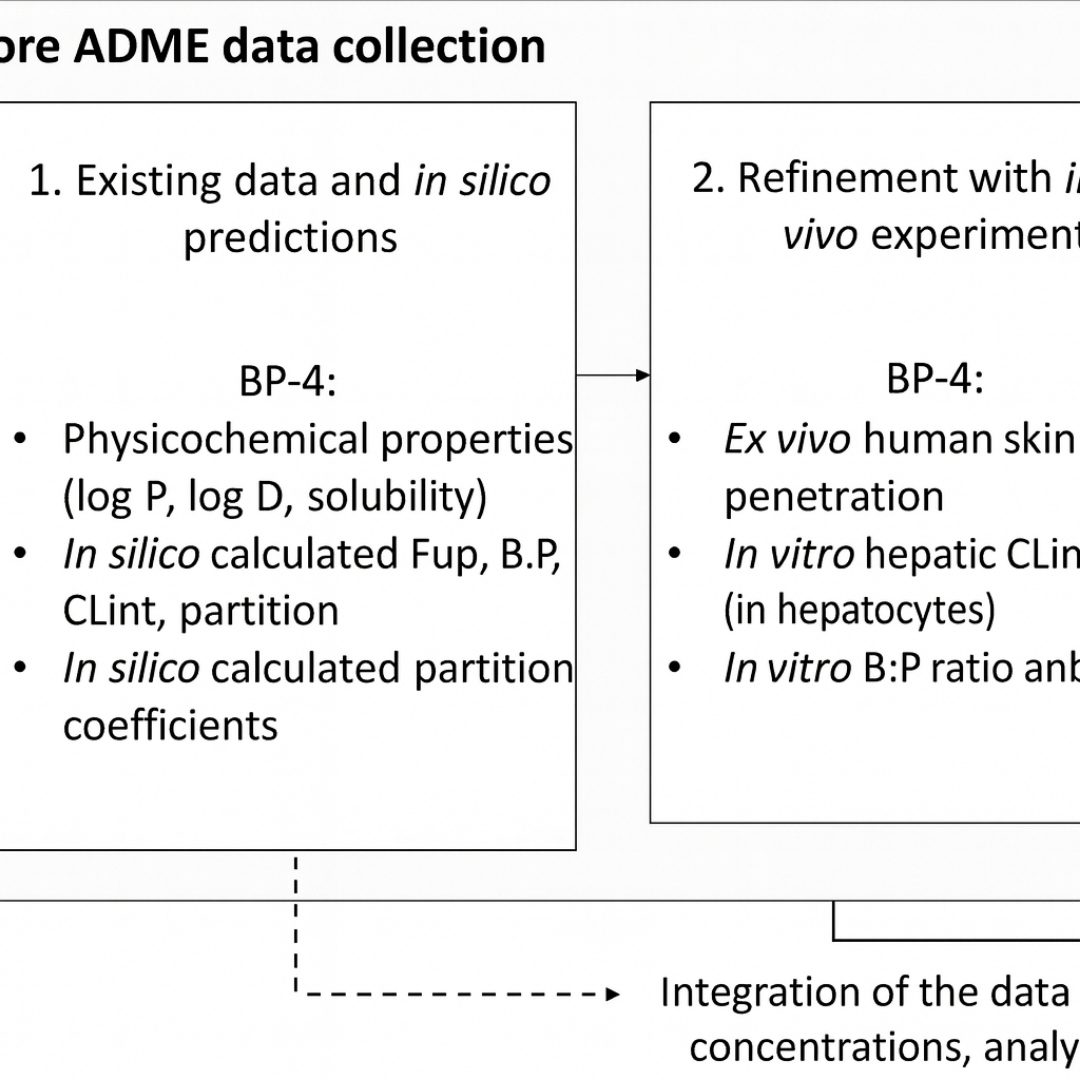
Building Confidence in PBK Model Predictions in the Absence of Human Kinetic Data: Benzophenone-4 Case Study
This study aimed to develop a physiologically based kinetic (PBK) model for benzophenone-4 (BP-4) in humans based on in vitro and in silico input data and to achieve scientific confidence in predicted internal...
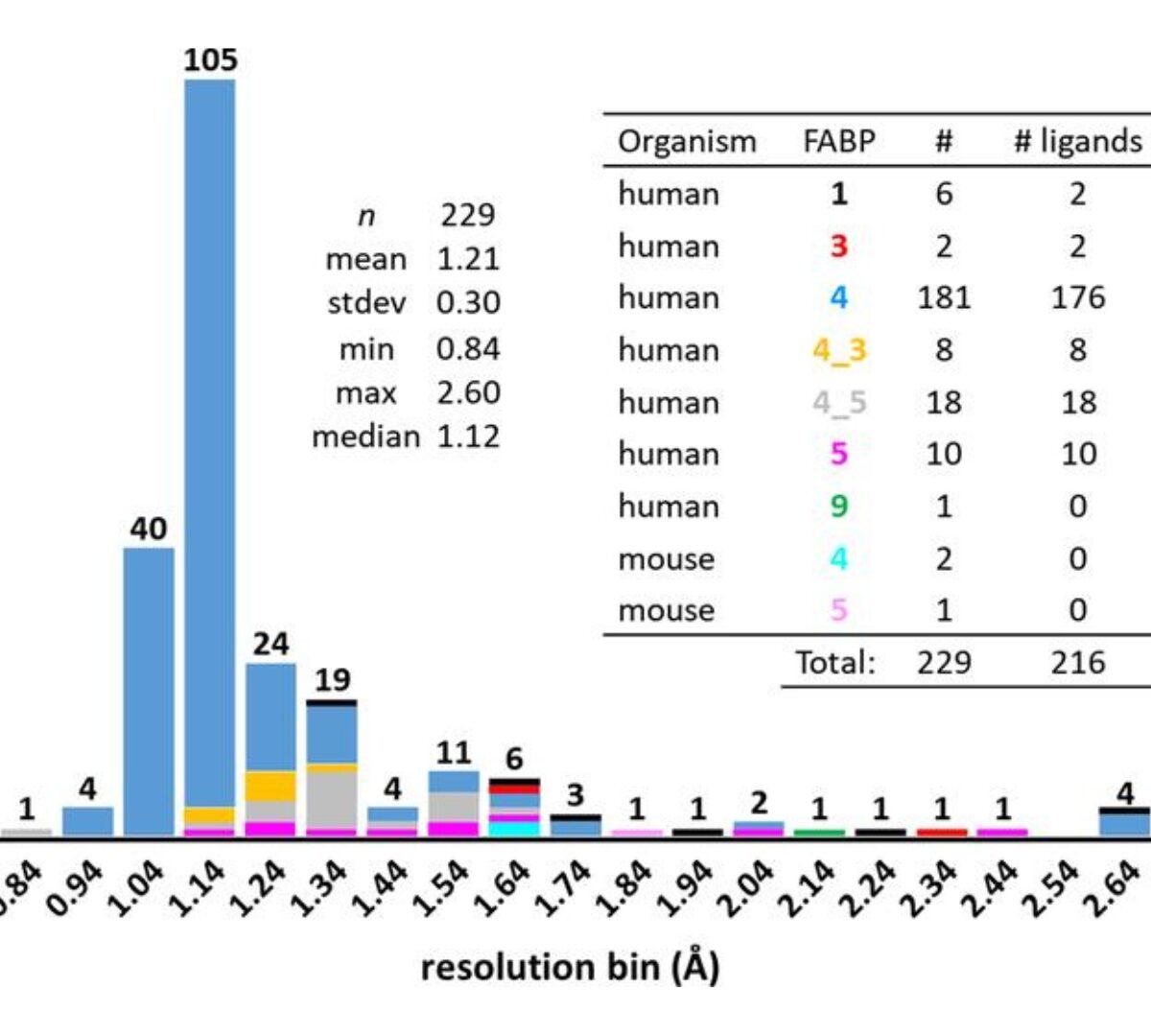
A High-Resolution Data Set of Fatty Acid-Binding Protein Structures. II. Crystallographic Overview, Ligand Classes and Binding Pose
Fatty acid-binding proteins (FABPs) belong to the calycin superfamily of proteins, sharing a similar overall structure with a ten-stranded β-barrel that encloses a large interior cavity for fatty-acid binding.
