Pimavanserin is a selective serotonin 5-HT2A receptor inverse agonist/antagonist being investigated in patients with negative symptoms of schizophrenia. This analysis aimed to characterize exposure-response relationships of...

Evaluating the bioequivalence of two pitavastatin calcium formulations based on IVIVC modeling and clinical study
In vitro-in vivo correlation (IVIVC) allows prediction of the in vivo performance of a pharmaceutical product based on its in vitro drug release profiles...

Predictive Dissolution Models for Real-Time Release Testing: Development and Implementation – Workshop Summary Report
To date, few examples of dissolution models for real-time release testing (RTRT) have been approved for commercial drug products or published in literature.

Can We Predict Clinical Pharmacokinetics of Highly Lipophilic Compounds by Integration of Machine Learning or In Vitro Data into Physiologically Based Models? A Feasibility Study Based on 12 Development Compounds
While high lipophilicity tends to improve potency, its effects on pharmacokinetics (PK) are complex and often unfavorable.

Clinical Ocular Exposure Extrapolation for Ophthalmic Solutions Using PBPK Modeling and Simulation
The development of generic ophthalmic drug products is challenging due to the complexity of the ocular system, and a lack of sensitive testing to evaluate the interplay of physiology with ophthalmic formulations.

Results from a Validated in vitro Gastrointestinal Model (TIM) used as input Data for in silico Modeling Give Highly Predictive Information for the Human Situation
The aim of this review paper is to evaluate the predictive quality of a combination of in vitro dynamic gastrointestinal models, mucosal transit models and in silico kinetic...

Translational pharmacokinetics of a novel bispecific antibody against Ebola virus (MBS77E) from animal to human by PBPK modeling & simulation
The goal of this study was to construct a PBPK model to accelerate the translation of MBS77E, a humanized bispecific antibody against the Ebola virus.

Investigating the uncertainty of prediction accuracy for the application of physiologically based pharmacokinetic models to animal-free risk assessment of cosmetic ingredients
Physiologically based pharmacokinetic (PBPK) models are considered useful tools in animal-free risk assessment.

Gastrointestinal Fluid Volumes in Pediatrics: A Retrospective MRI Study
The volume and distribution of fluids available in the gastrointestinal (GI) tract may substantially affect oral drug absorption.

Utility of Physiologically Based Biopharmaceutics Modeling (PBBM) in Regulatory Perspective: Application to Supersede f2, Enabling Biowaivers & Creation of Dissolution Safe Space
Product DRL is a generic IR tablet formulation with BCS Class-III API, available in two strengths: 50mg & 100mg.

Towards best use and regulatory acceptance of generic physiologically based kinetic (PBK) models for in vitro-to-in vivo extrapolation (IVIVE) in chemical risk assessment
With an increasing need to incorporate new approach methodologies (NAMs) in chemical risk assessment and the concomitant need to phase out animal testing, the interpretation of...

A novel transdermal ketoprofen formulation for analgesia in cattle
Ketoprofen is registered in many countries for injectable administration in cattle.

Physiologically based Pharmacokinetic Models under the Prism of the Finite Absorption Time Concept
To date, mechanistic modeling of oral drug absorption has been achieved via the use of physiologically based pharmacokinetic (PBPK) modeling, and more specifically, physiologically...
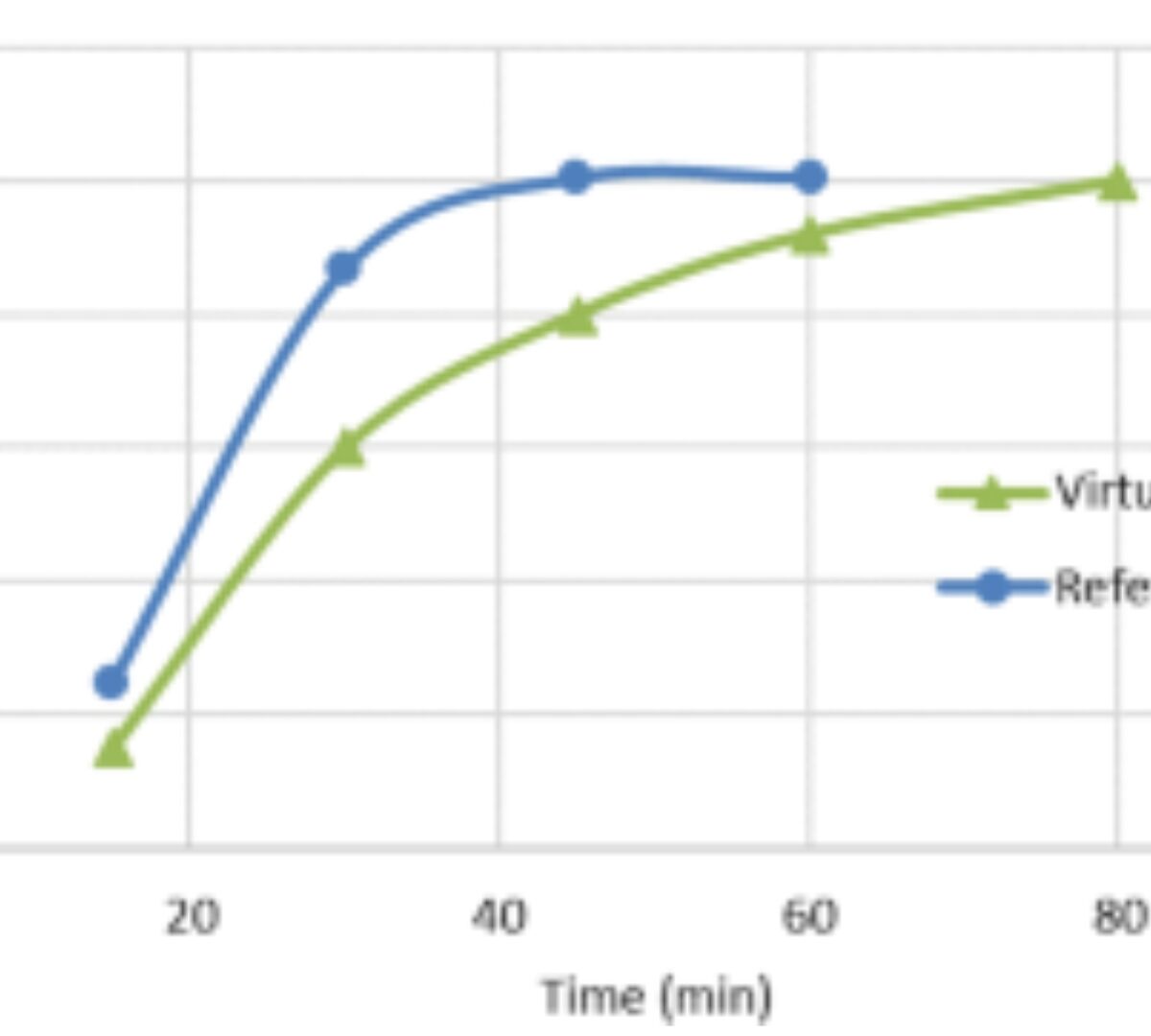
Physiologically Based Biopharmaceutics Model for Selumetinib Food Effect Investigation and Capsule Dissolution Safe Space – Part I: Adults
A physiologically based biopharmaceutics model (PBBM) was developed to mechanistically investigate the effect of formulation and food on selumetinib pharmacokinetics.

Metabolism of 3-Chlorobiphenyl (PCB 2) in a Human-Relevant Cell Line: Evidence of Dechlorinated Metabolites
Lower chlorinated polychlorinated biphenyls (LC-PCBs) and their metabolites make up a class of environmental pollutants implicated in a range of adverse outcomes in...

Simulation of Intraluminal Performance of Lipophilic Weak Bases in Fasted Healthy Adults Using DDDPlusTM
The majority of drug candidates exhibit weakly basic characteristics with high lipophilicity.

Simulation of Intraluminal Performance of Lipophilic Weak Bases in Fasted Healthy Adults Using DDDPlusTM
The majority of drug candidates exhibit weakly basic characteristics with high lipophilicity. The risk of intraluminal compound precipitation has been studied in vivo...

Physiologically based pharmacokinetic modeling of daptomycin dose optimization in pediatric patients with renal impairment
Daptomycin is used to treat Gram-positive infections in adults and children and its dosing varies among different age groups.

In Vitro–In Vivo Relationship in Mini-Scale—Enabling Formulations of Corallopyronin A
In vivo studies in mice provide a valuable model to test novel active pharmaceutical ingredients due to their low material need and the fact that mice are frequently used as a species...

Reliance is key to effective access and oversight of medical products in case of public health emergencies
Responding to new threats and public health emergencies (PHE) creates serious challenges to regulators.
