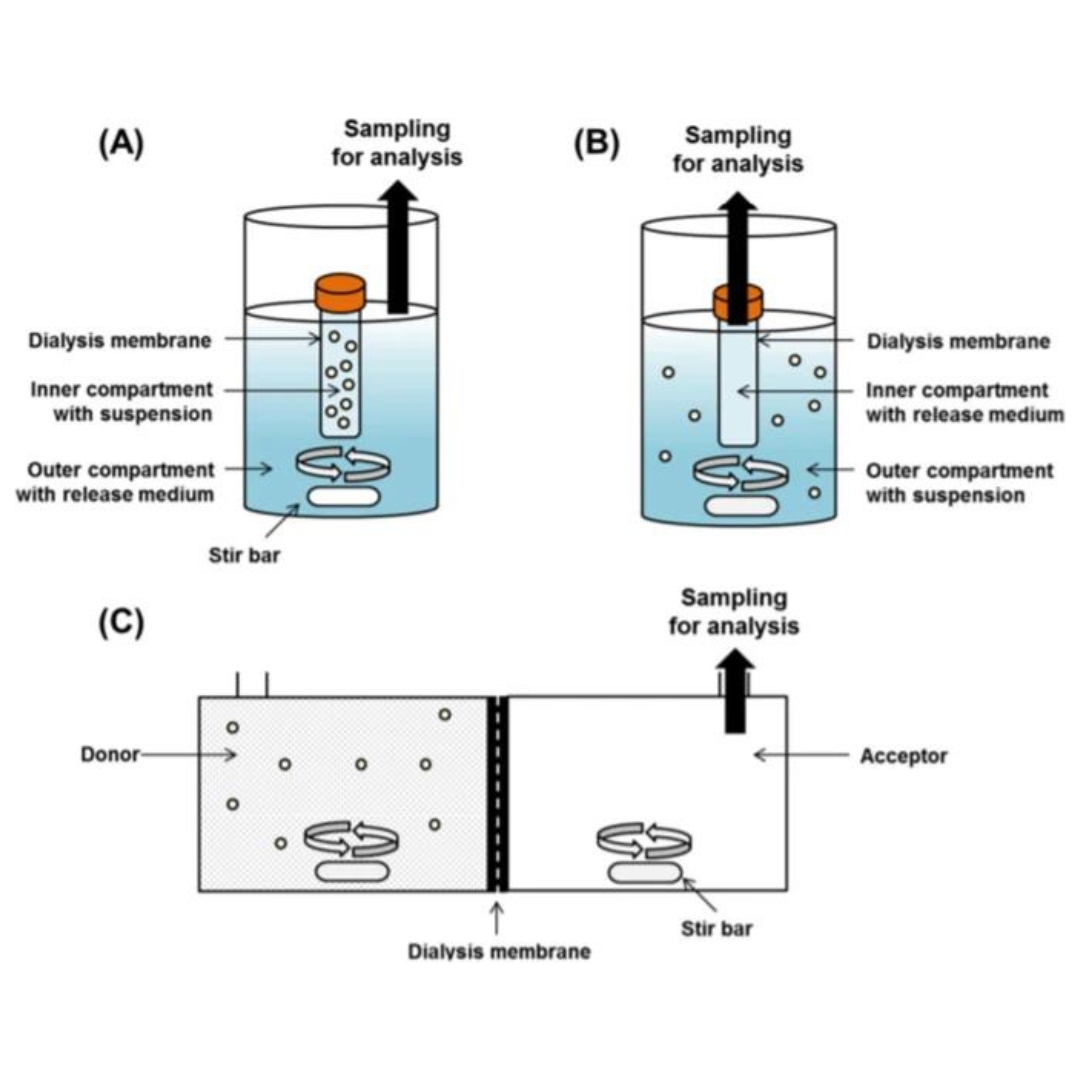
Long-acting injectable (LAI) formulations can provide several advantages over the more traditional oral formulation as drug product opportunities. LAI formulations...
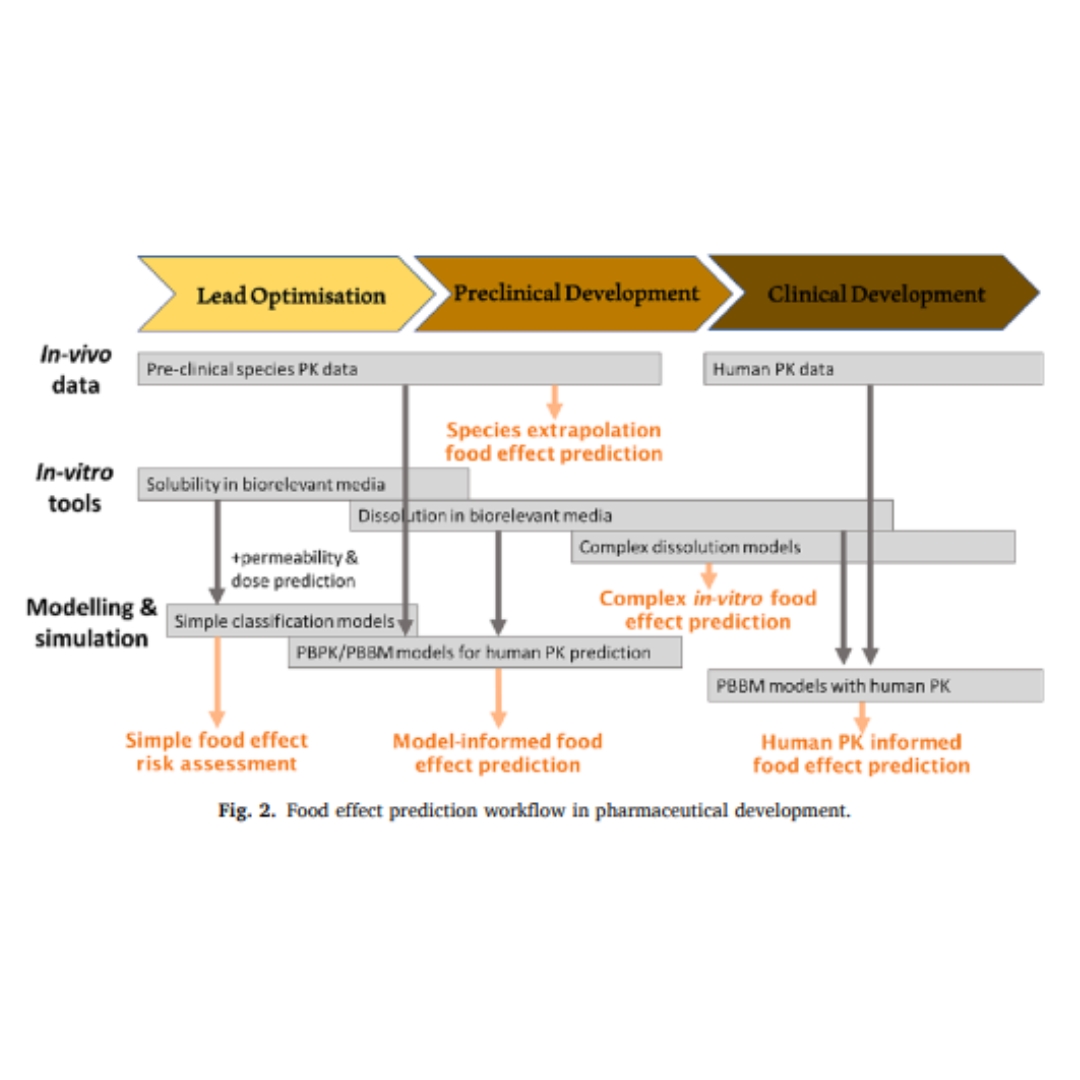
Assessment of food effects during clinical development
Food-drug interactions frequently hamper oral drug development due to various physicochemical, physiological and formulation-dependent mechanisms.

Exploring the Use of a Kinetic pH Calculation to Correct the ACAT Model with a Single Stomach Compartment Setting: Impact of Stomach Setting on Food Effect Prediction for Basic Compounds
Advanced compartmental absorption and transit (ACAT) based computational models have become increasingly popular in the industry for predicting oral drug product...
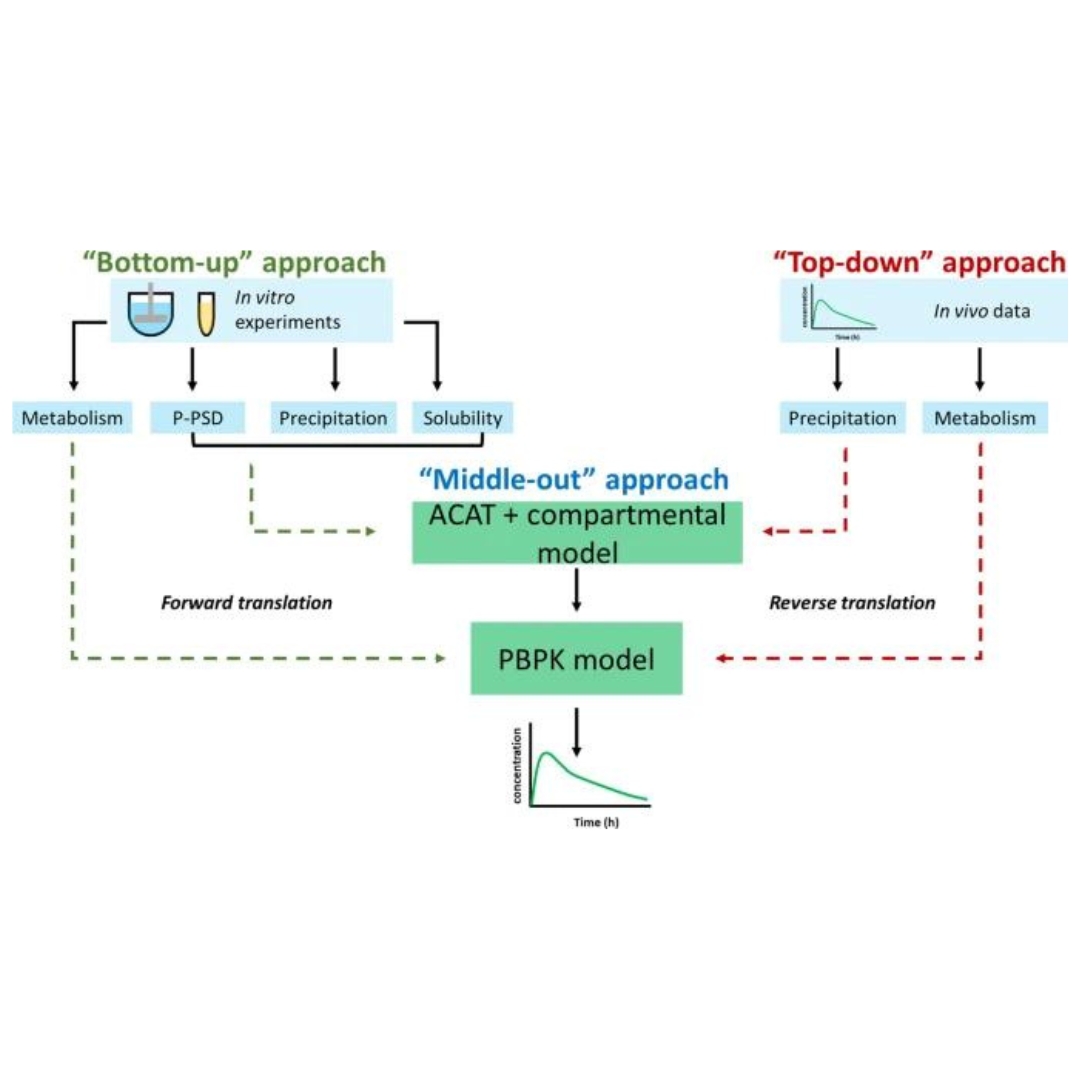
Integrating Forward and Reverse Translation in PBPK Modeling to Predict Food Effect on Oral Absorption of Weakly Basic Drugs
Ketoconazole and posaconazole are two weakly basic broad-spectrum antifungals classified as Biopharmaceutics Classification System class II drugs, indicating that they are...
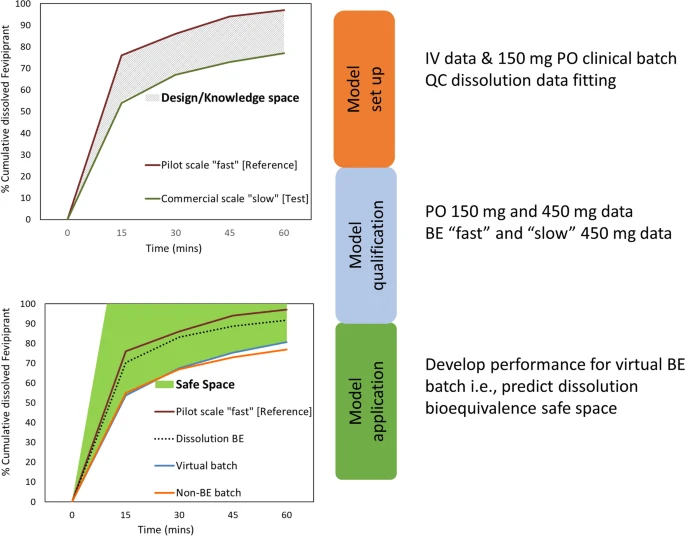
Establishing the Safe Space via Physiologically Based Biopharmaceutics Modeling. Case Study: Fevipiprant/QAW039
Physiologically based pharmacokinetic and absorption modeling has increasingly been implemented for biopharmaceutics applications to define the safe space for drug...
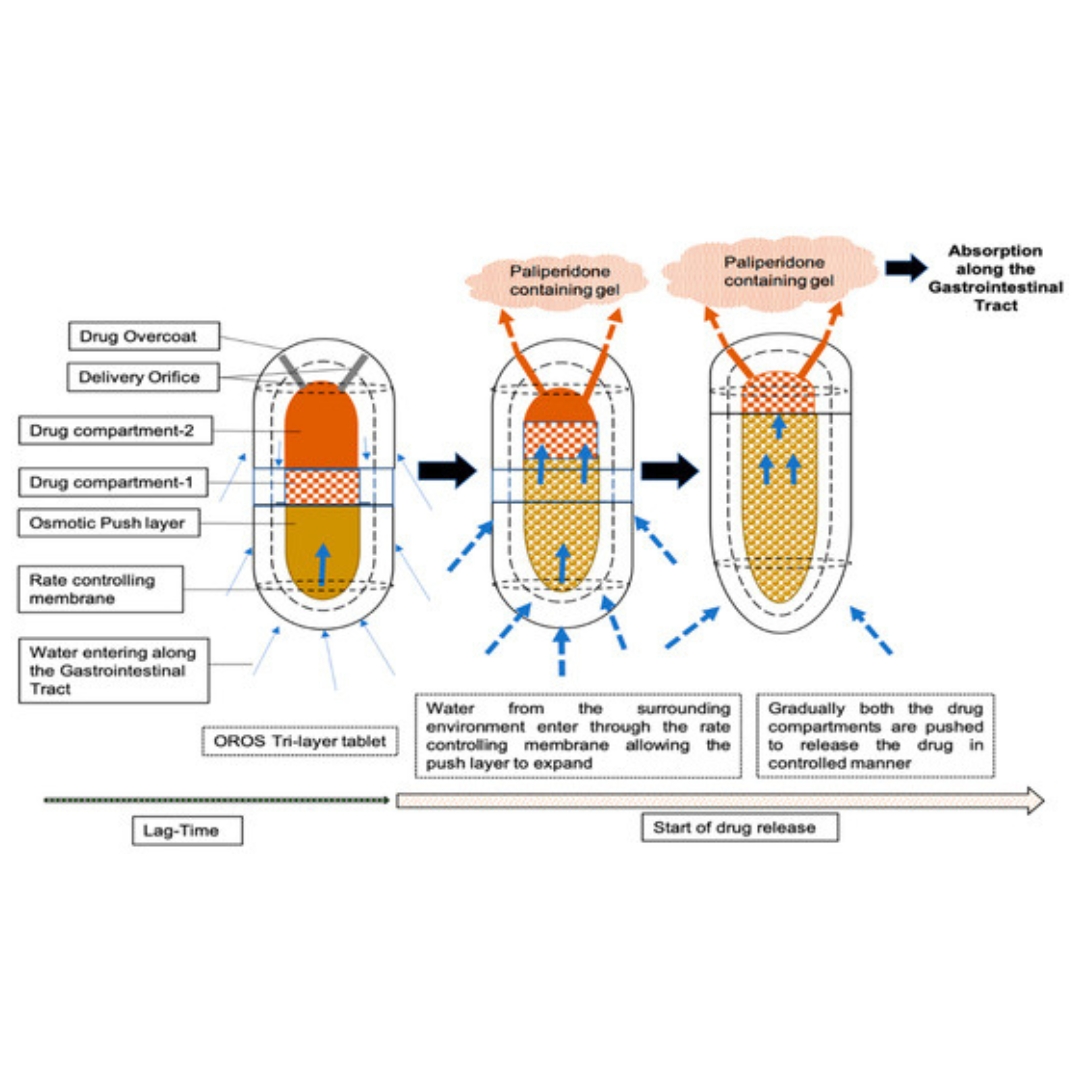
Leveraging Physiologically Based Modelling to Provide Insights on the Absorption of Paliperidone Extended-Release Formulation under Fed and Fasting Conditions
Paliperidone was approved by the US FDA in 2006 as an extended-release (ER) tablet (Invega®) for the once-daily treatment of schizophrenia. This osmotic-controlled...

Regulatory Requirements for the Development of Second-Entry Semisolid Topical Products in the European Union
The development of second-entry topical products is hampered by several factors.
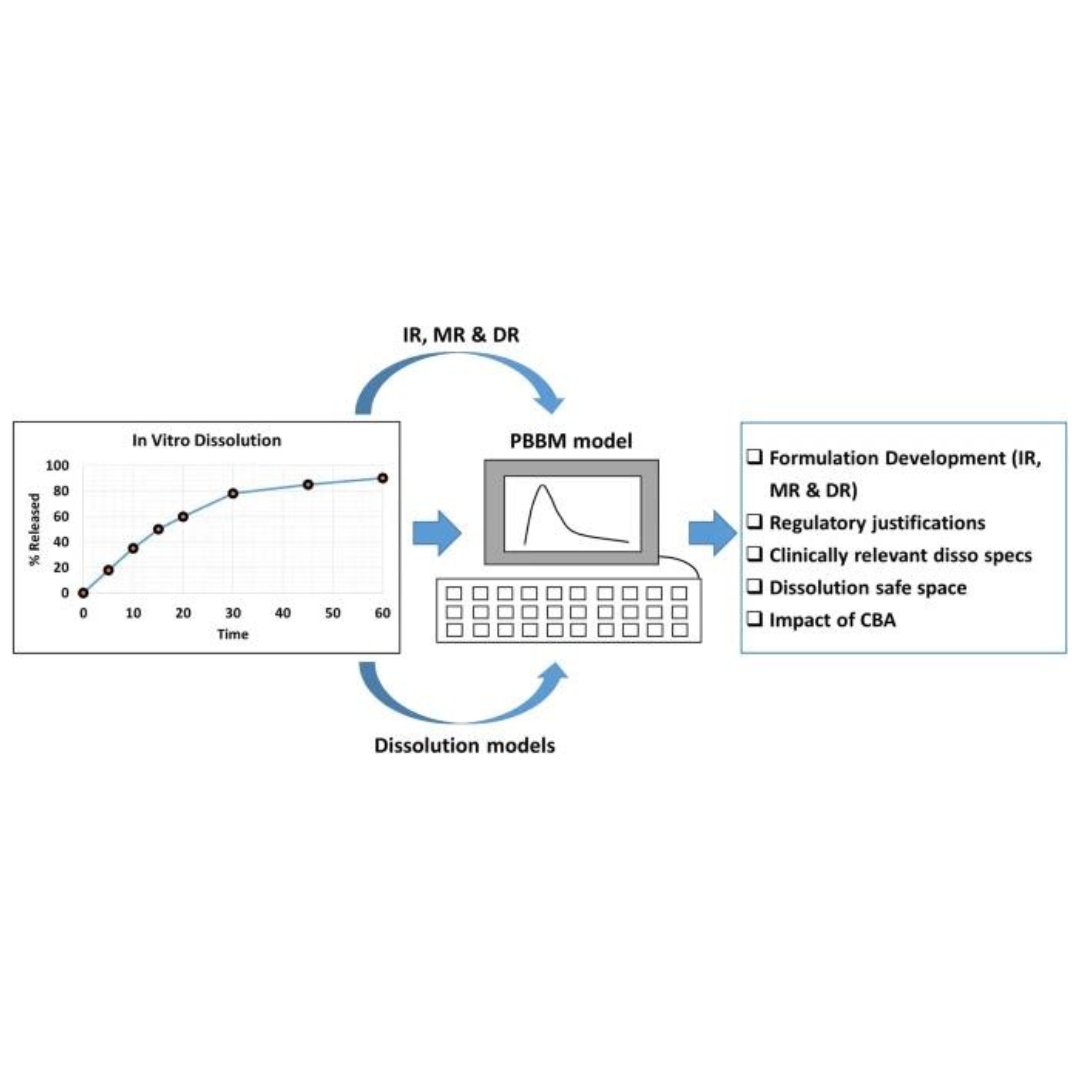
Best Practices for Integration of Dissolution Data into Physiologically Based Biopharmaceutics Models (PBBM): A Biopharmaceutics Modeling Scientist Perspective
Dissolution is considered as a critical input into physiologically based biopharmaceutics models (PBBM) as it governs in vivo exposure. Despite many workshops, initiatives...

Addressing the oxamniquine in vitro-in vivo paradox to facilitate a new generation of anti-schistosome treatments
The antischistosomal drug oxamniquine, OXA, requires activation by a sulfotransferase within the parasitic worm to enable killing.

An Insight into the Metabolism of 2,5-Disubstituted Monotetrazole Bearing Bisphenol Structures: Emerging Bisphenol A Structural Congeners
The non-estrogenic 2,5-disubstituted tetrazole core-bearing bisphenol structures (TbB) are being researched as emerging structural congeners of Bisphenol...

An innovative impurity profiling of esmolol hydrochloride injection using UPLC-MS based multiple mass defect filter, chemometrics and in-silico toxicity prediction
Esmolol hydrochloride injection is indicated for the rapid control of ventricular rate in patients with atrial fibrillation or atrial flutter in perioperative...
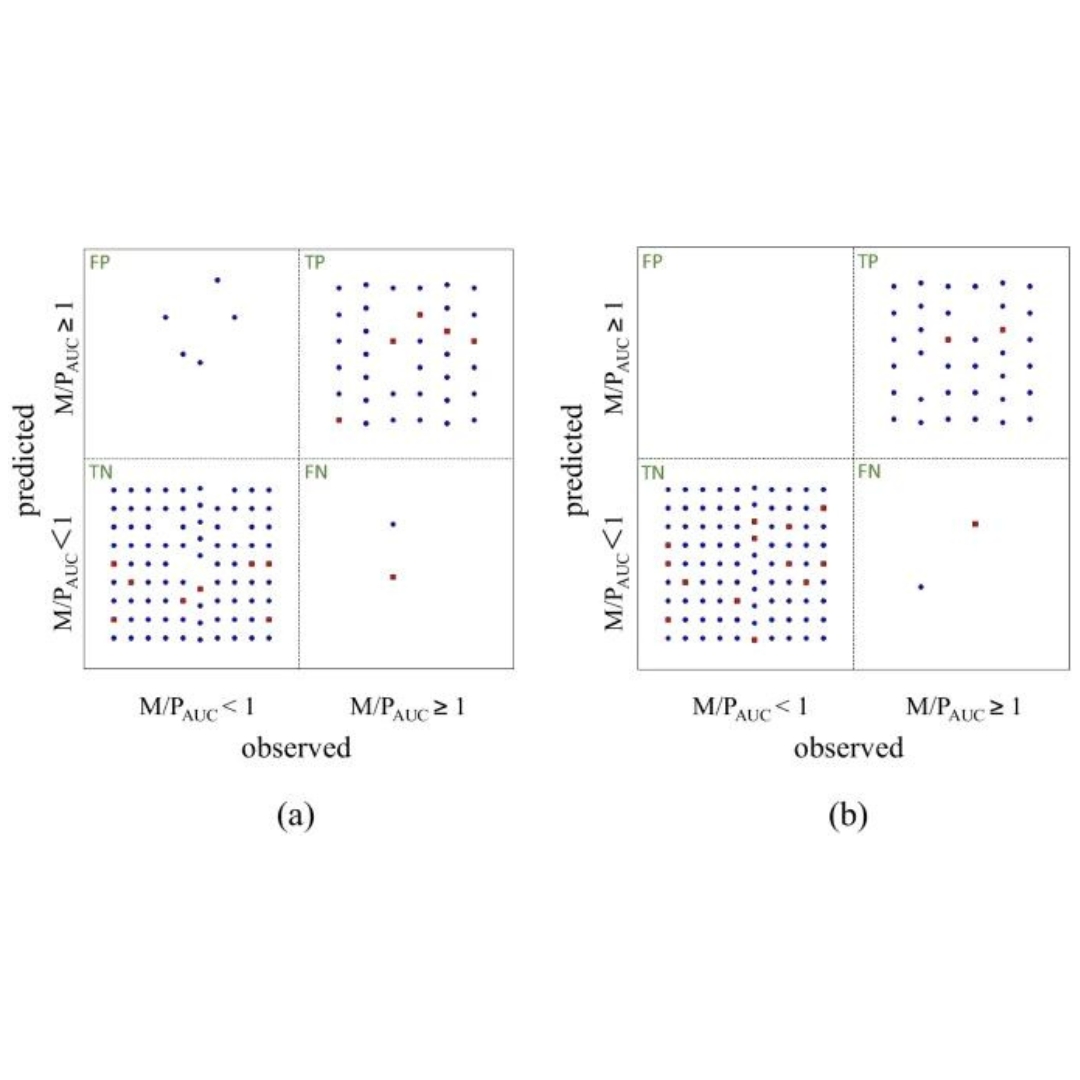
Prediction model for milk transfer of drugs by primarily evaluating the area under the curve using QSAR/QSPR
Information on milk transferability of drugs is important for patients who wish to breastfeed. The purpose of this study is to develop a prediction model for milk-to-plasma...

Alternative Pharmacokinetic Metrics in Single-Dose Studies to Ensure Bioequivalence of Prolonged-Release Products at Steady State-A Case Study
This article investigates which PK metrics in a single-dose study (con-centration at the end of posology interval, Cτ, partial areas under the curve, pAUCs, or half-valueduration, HVD) are more sensitive and less variable...
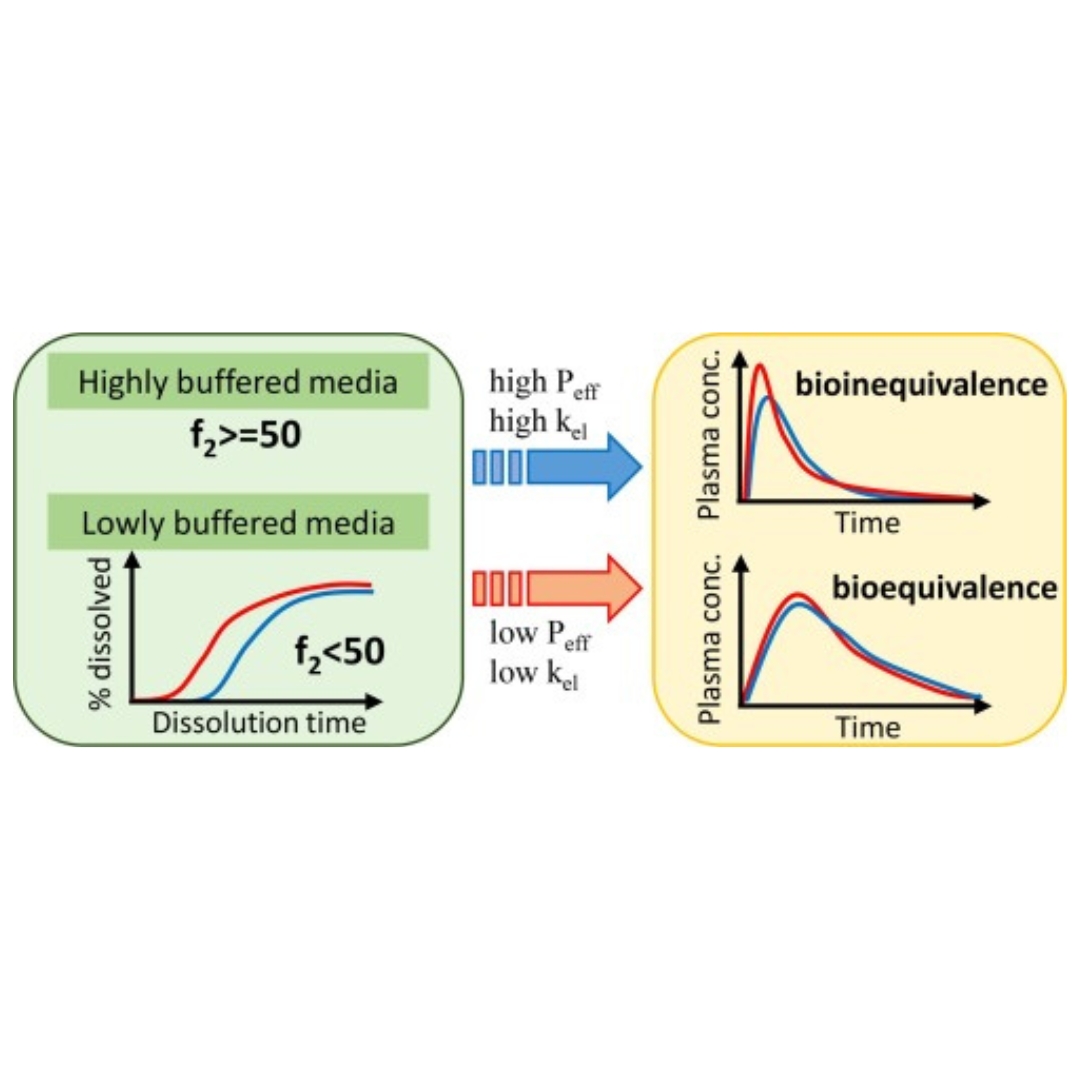
Lowly-buffered biorelevant dissolution testing is not necessarily biopredictive of human bioequivalence study outcome: Relationship between dissolution and pharmacokinetics
It has been revealed that buffer capacity of aspirated human intraluminal fluid is much lower than that of in vitro compendial dissolution media. Since buffer capacity signif
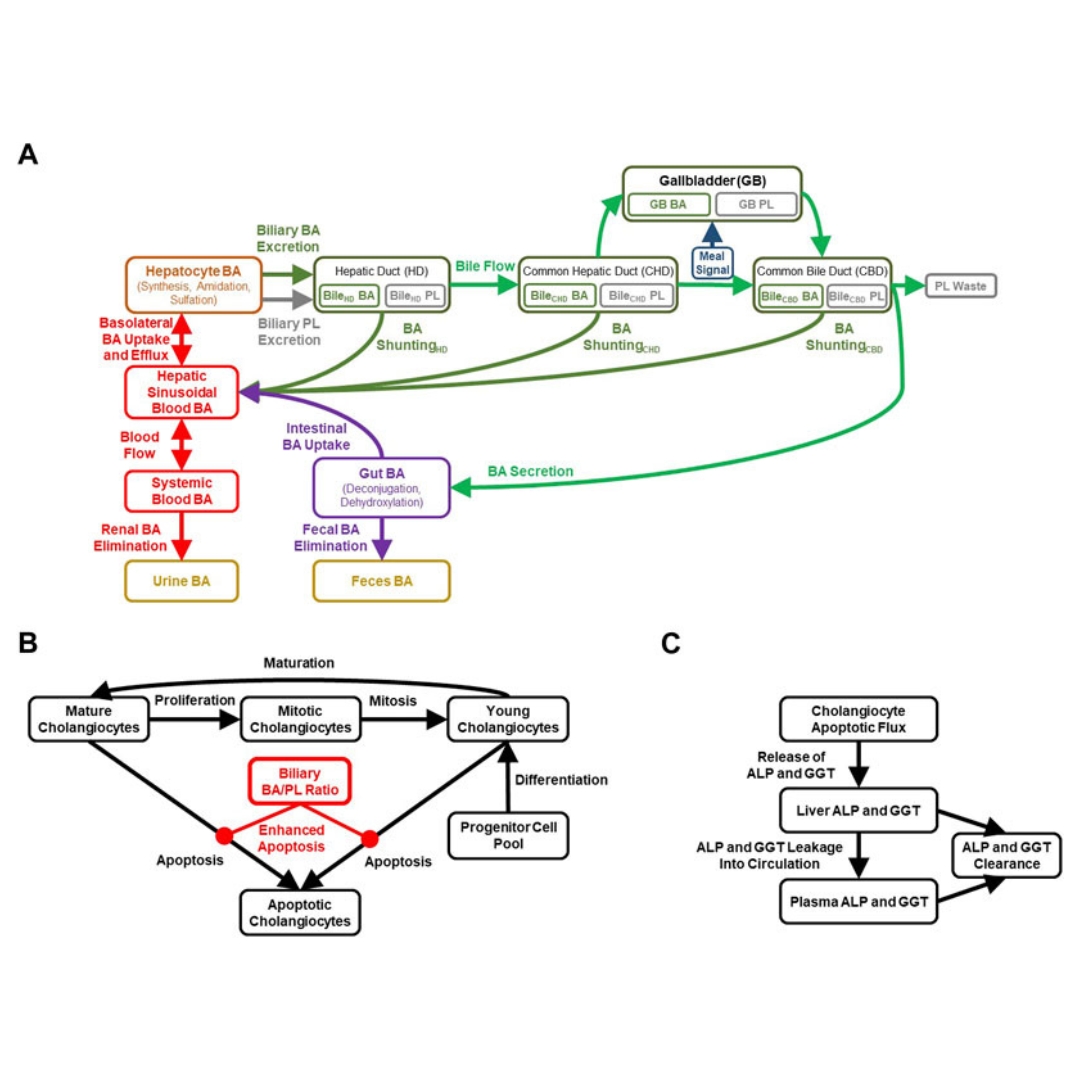
Investigating bile acid-mediated cholestatic drug-induced liver injury using a mechanistic model of multidrug resistance protein 3 (MDR3) inhibition
Inhibition of the canalicular phospholipid floppase multidrug resistance protein 3 (MDR3) has been implicated in cholestatic drug-induced liver injury (DILI), which is clinically...

N-Derivatives of (Z)-Methyl 3-(4-Oxo-2-thioxothiazolidin-5-ylidene)methyl)-1H-indole-2-carboxylates as Antimicrobial Agents—In Silico and In Vitro Evaluation
Herein, we report the experimental evaluation of the antimicrobial activity of seventeen new (Z)-methyl 3-(4-oxo-2-thioxothiazolidin-5-ylidene)methyl)-1H-indole-2-carboxylate derivatives.
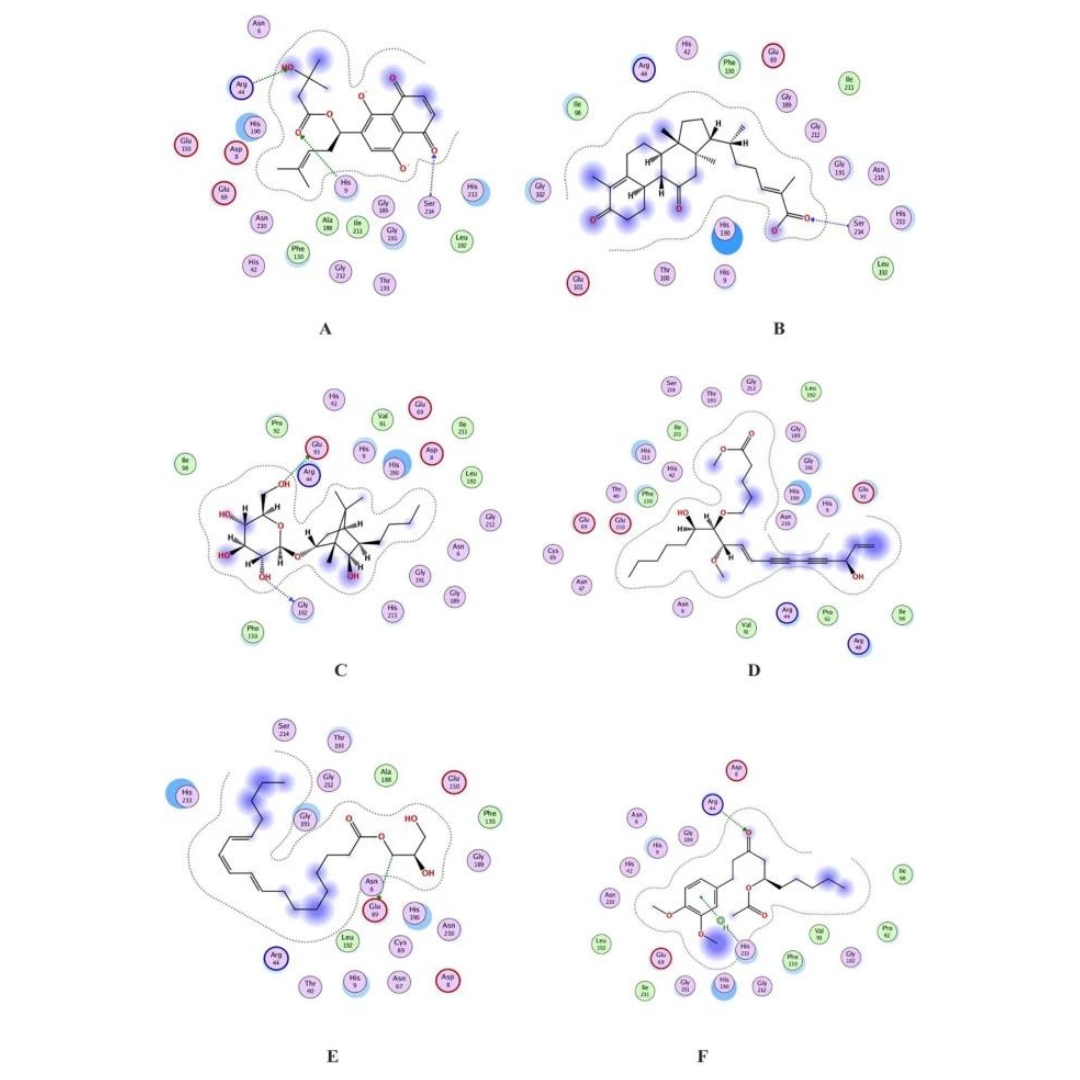
Therapeutic target mapping from the genome of Kingella negevensis and biophysical inhibition assessment through PNP synthase binding with traditional medicinal compounds
Kingella negevensis belongs to the Neisseriaceae family. It is implied that it has significant virulence potential due to RTX toxin production, which can cause hemolysis.
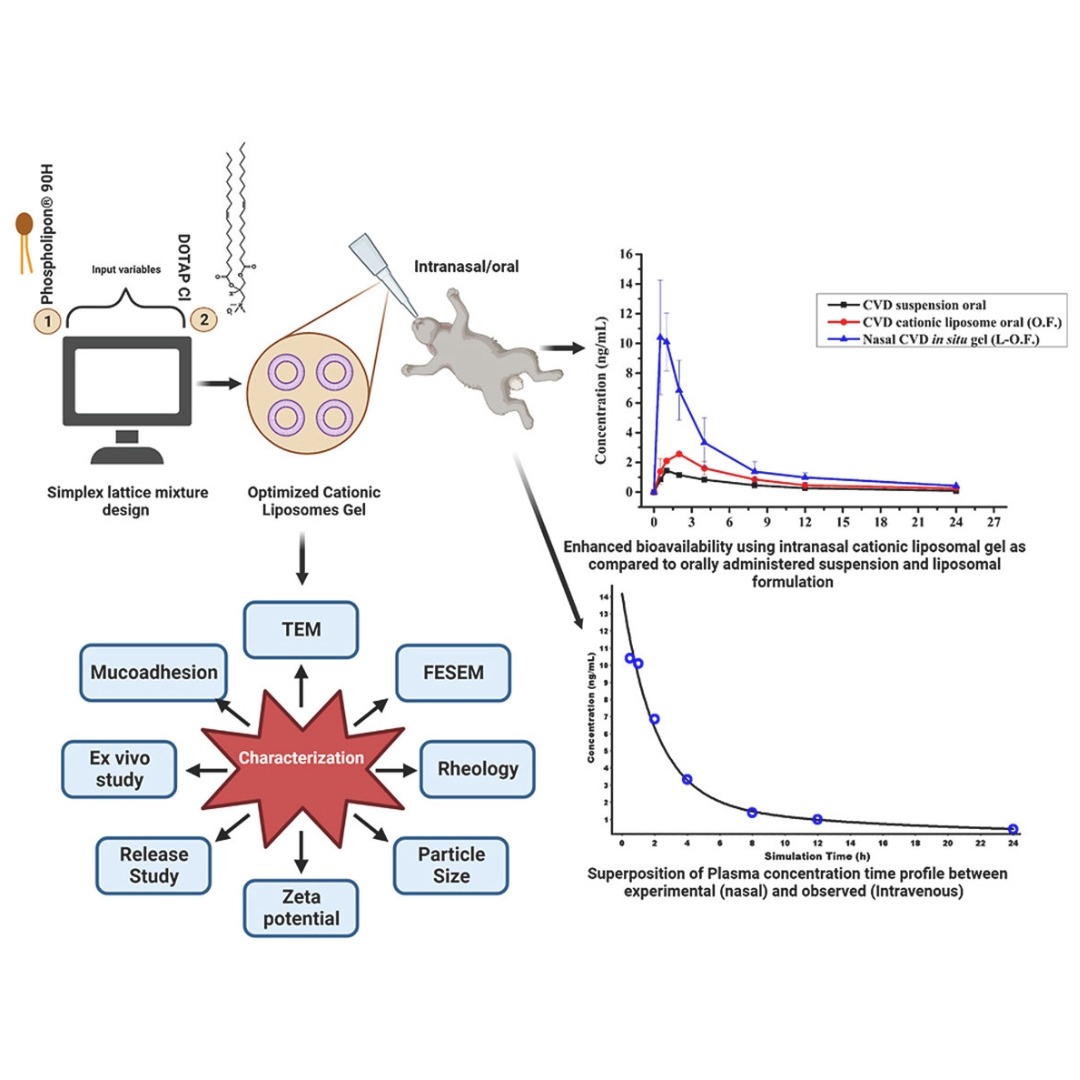
Cationic nanoliposomes of carvedilol for intranasal application: In vitro, in vivo and in silico studies
Carvedilol (CVD) is a non-selective β and α adrenoreceptor blocker, useful in treating hypertension, angina pectoris, congestive heart failure (CHF), and coronary artery...

Safety, Tolerability, and Pharmacokinetics of Nebulized Hydroxychloroquine: A Pilot Study in Healthy Volunteers
Background: Early in the coronavirus disease 2019 (COVID-19) pandemic, hydroxychloroquine (HCQ) drew substantial attention as a potential COVID-19 treatment...
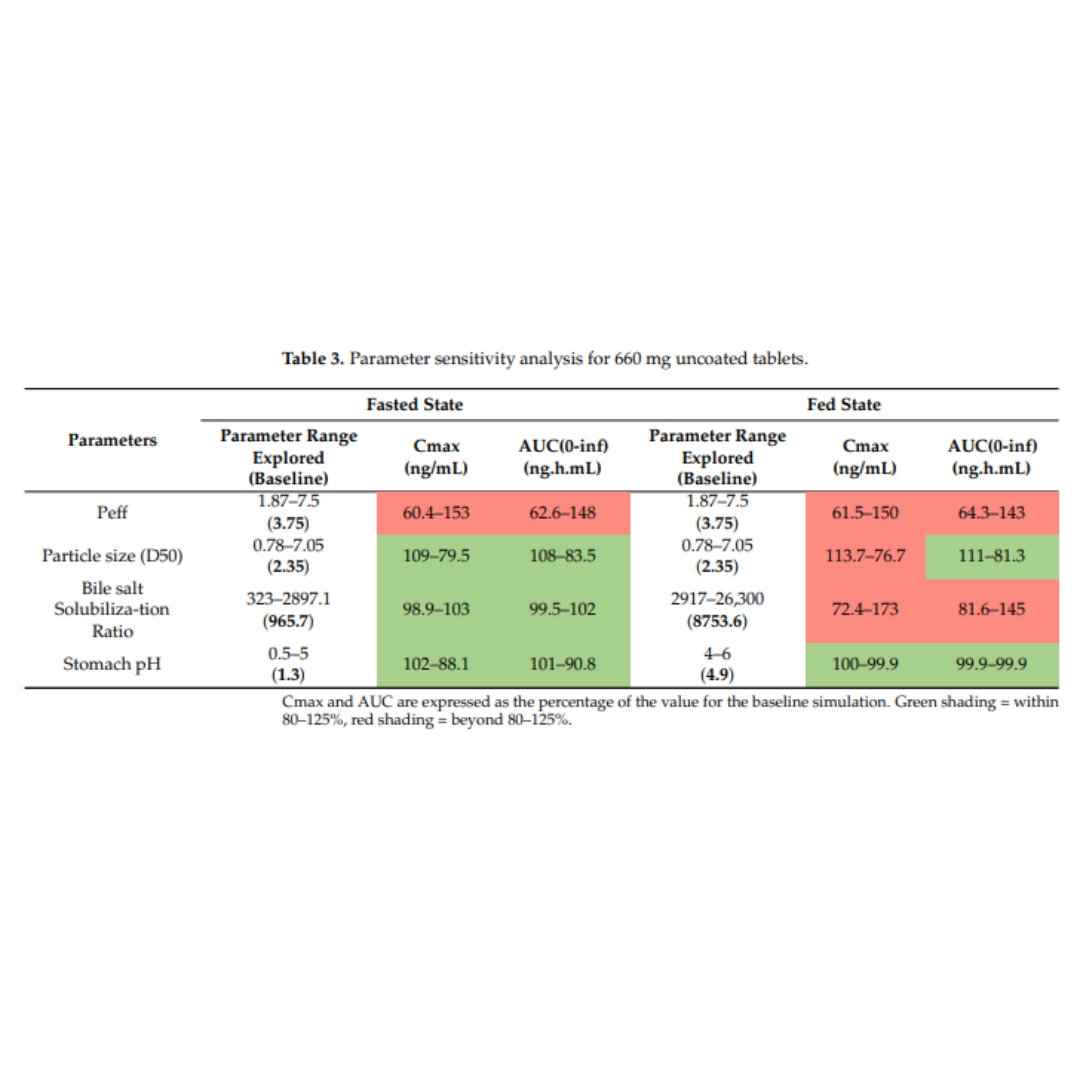
Physiologically Based Biopharmaceutics Modeling of Food Effect for Basmisanil: A Retrospective Case Study of the Utility for Formulation Bridging
Basmisanil, is a lipophilic drug substance, exhibiting poor solubility and good permeability (BCS class 2). A validated physiologically based biopharmaceutics model (PBBM)...