Oral vaccines represent many advantages compared to standard vaccines. They hold a simple method of administration and manufacturing process.
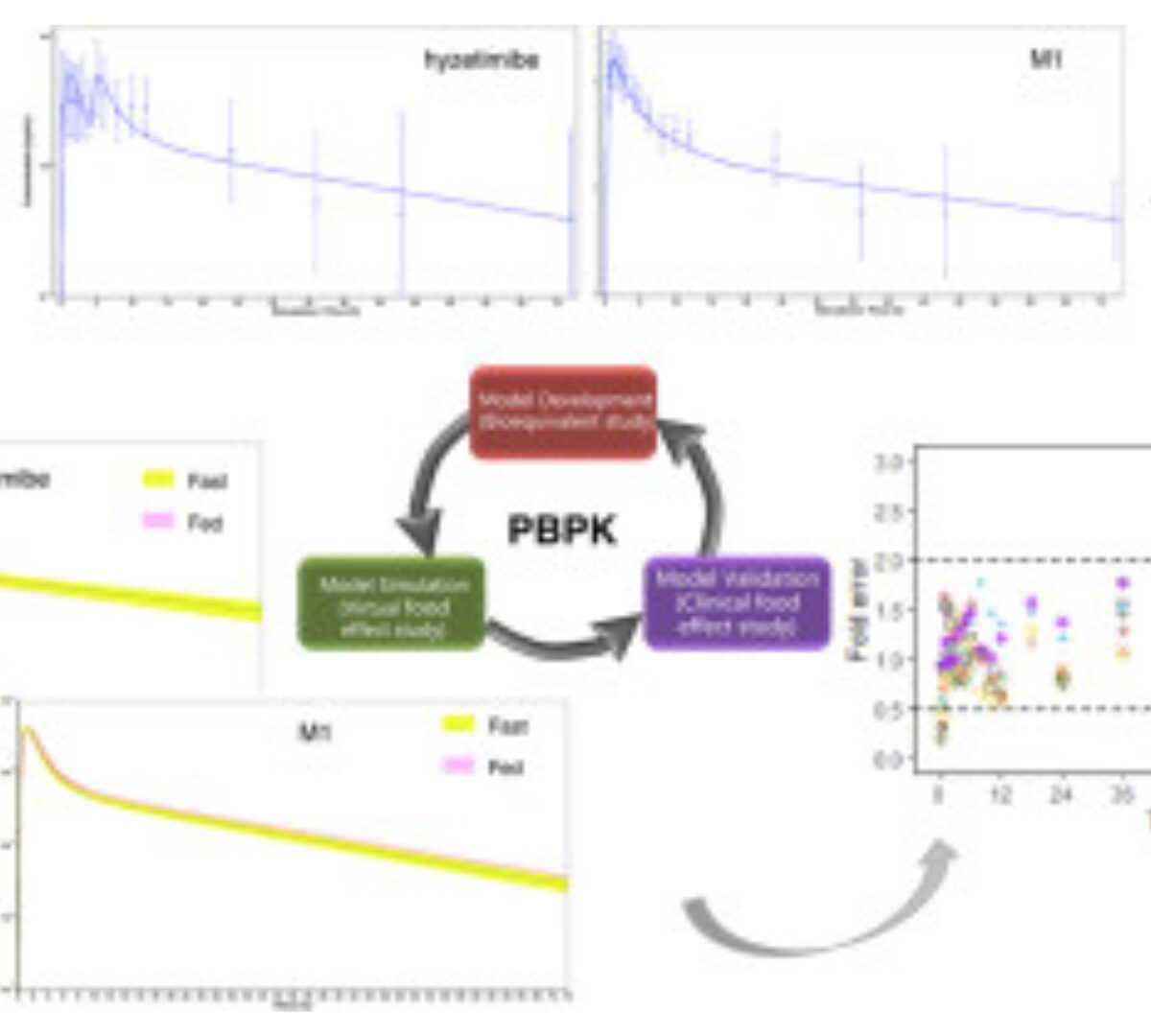
Physiologically based pharmacokinetic modeling to characterize enterohepatic recirculation and predict food effect on the pharmacokinetics of hyzetimibe
Hyzetimibe is a cholesterol absorption inhibitor indicated for the treatment of hypercholesterolemia.
![The anti-infective crotalicidin peptide analog RhoB-Ctn[1–9] is harmless to bovine oocytes and able to induce parthenogenesis in vitro](https://www.simulations-plus.com/wp-content/themes/simulations-plus/library/dist/img/default_square-large.jpg)
The anti-infective crotalicidin peptide analog RhoB-Ctn[1–9] is harmless to bovine oocytes and able to induce parthenogenesis in vitro
Crotalicidin is a cathelicidin-related anti-infective (antimicrobial) peptide expressed in the venom glands of the South American rattlesnake Crotalus durissus terrificus.
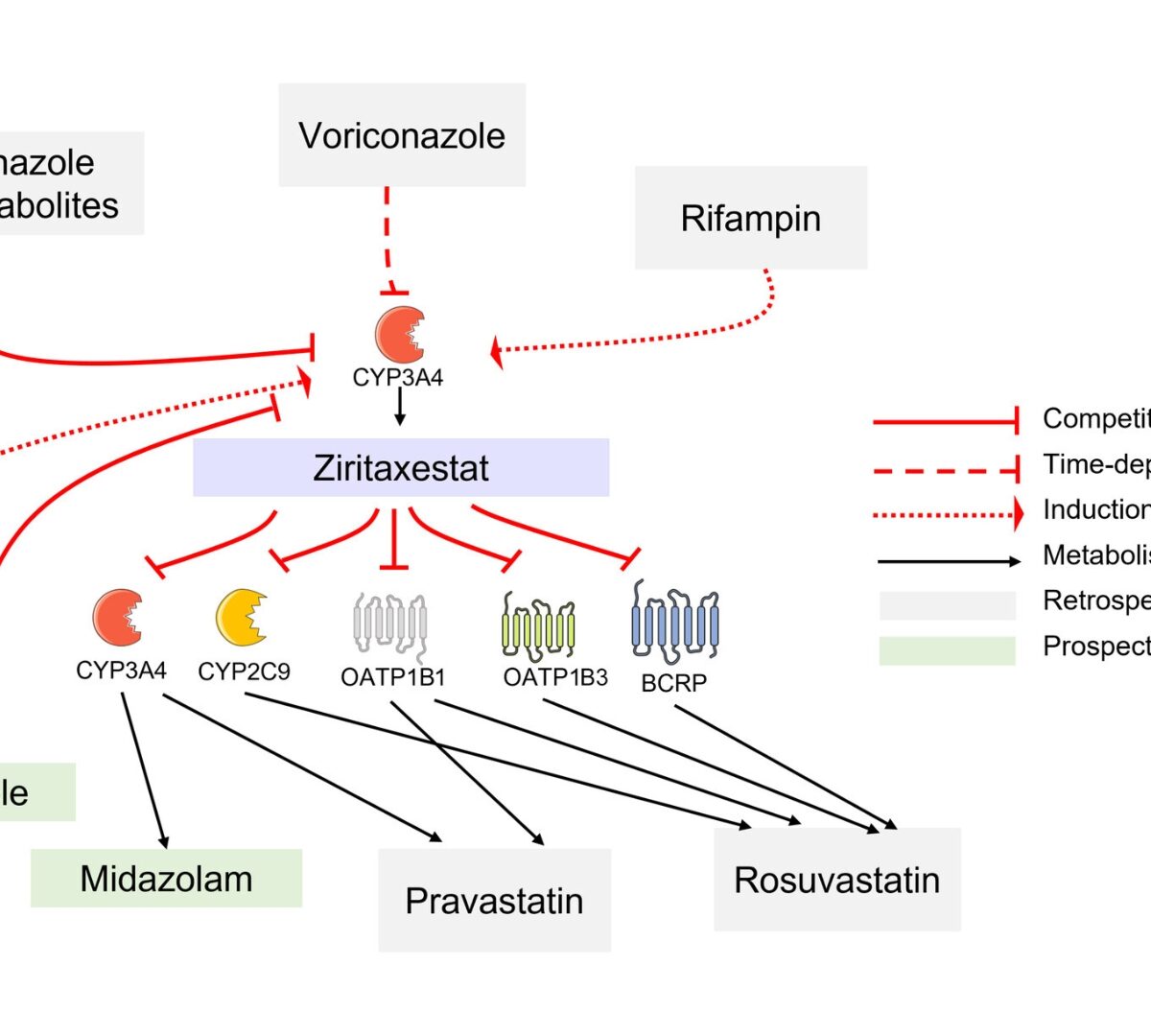
Drug–drug interaction prediction of ziritaxestat using a physiologically based enzyme and transporter pharmacokinetic network interaction model
Ziritaxestat, an autotaxin inhibitor, was under development for the treatment of idiopathic pulmonary fibrosis.
Physiologically based pharmacokinetic modelling to predict drug–drug interactions for encorafenib. Part I. Model building, validation, and prospective predictions with enzyme inhibitors, inducers, and transporter inhibitors
Encorafenib, a potent BRAF kinase inhibitor undergoes significant metabolism by CYP3A4 (83%) and CYP2C19 (16%) and also a substrate of P-glycoprotein (P-gp).

Mind the Gap: Model-Based Switching from Selatogrel to Maintenance Therapy with Oral P2Y12 Receptor Antagonists
The P2Y12 receptor antagonist selatogrel is being developed for subcutaneous self-administration with a ready-to-use autoinjector at the onset of acute myocardial infarction (AMI)symptoms.
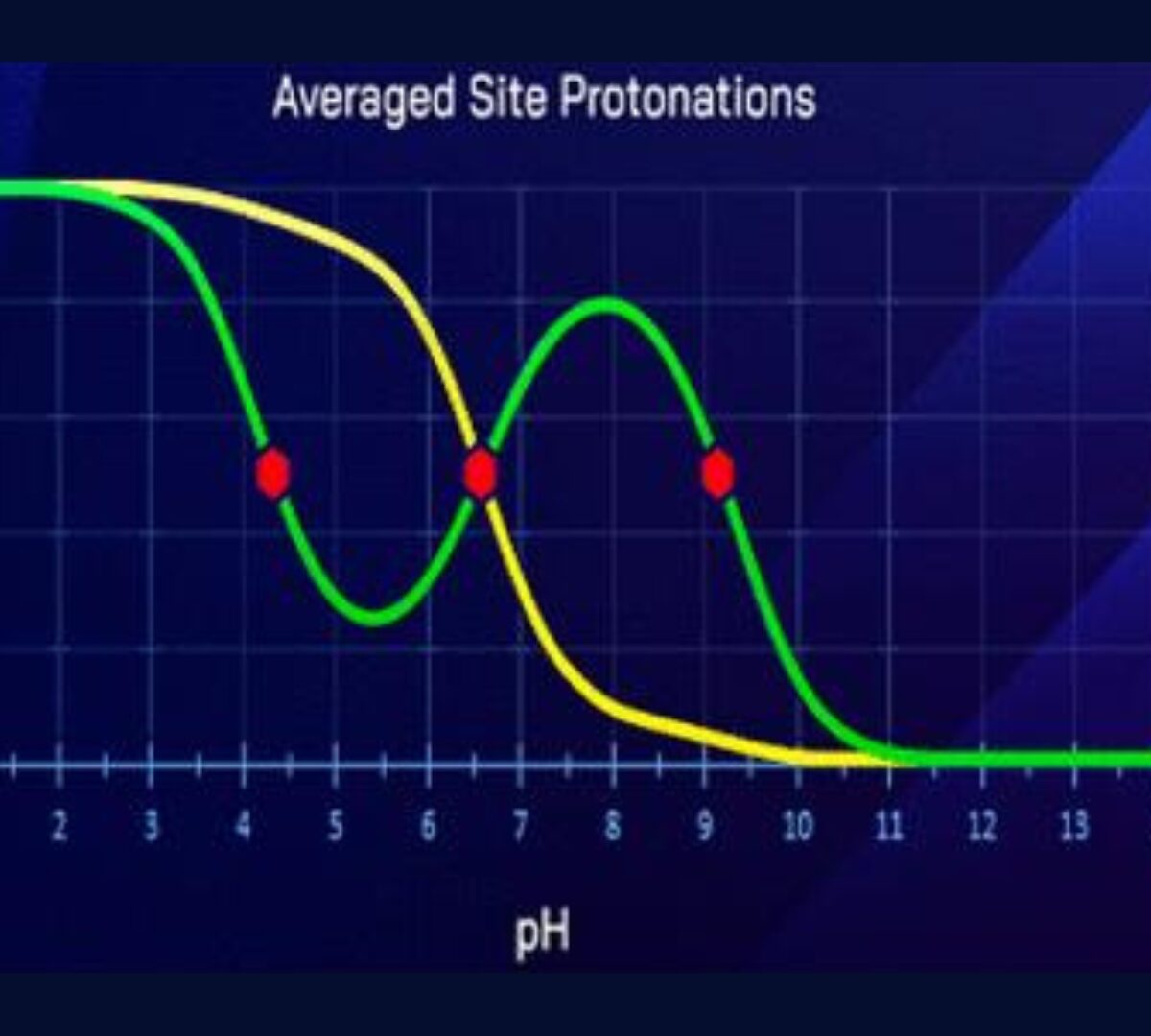
Can an Amine Be a Weaker and a Stronger Base at the Same Time? Curious Cases of Chameleonic Ionization
We discovered an anomalous basic dissociation in certain multiprotic compounds.
Dabigatran Dosing Proposal for Adults With Atrial Fibrillation: Stress-Testing Renal Function Range in Real World Patients
Dabigatran is the first of four direct-acting oral anticoagulants approved to prevent stroke in adult patients with atrial fibrillation using a fixed two-dose scheme compared with warfarin dosing adjusted to...
Physiologically based pharmacokinetic modeling (PBPK) to predict drug-drug interactions for encorafenib. Part II. Prospective predictions in hepatic and renal impaired populations with clinical inhibitors and inducers
Encorafenib, a potent BRAF kinase inhibitor gets significantly metabolised by CYP3A4 (83%) and CYP2C19 (16%) and is a substrate for P-glycoprotein...

Pyronaridine: a review of its clinical pharmacology in the treatment of malaria
Pyronaridine-artesunate was recently strongly recommended in the 2022 update of the WHO Guidelines for the Treatment of Malaria, becoming the...
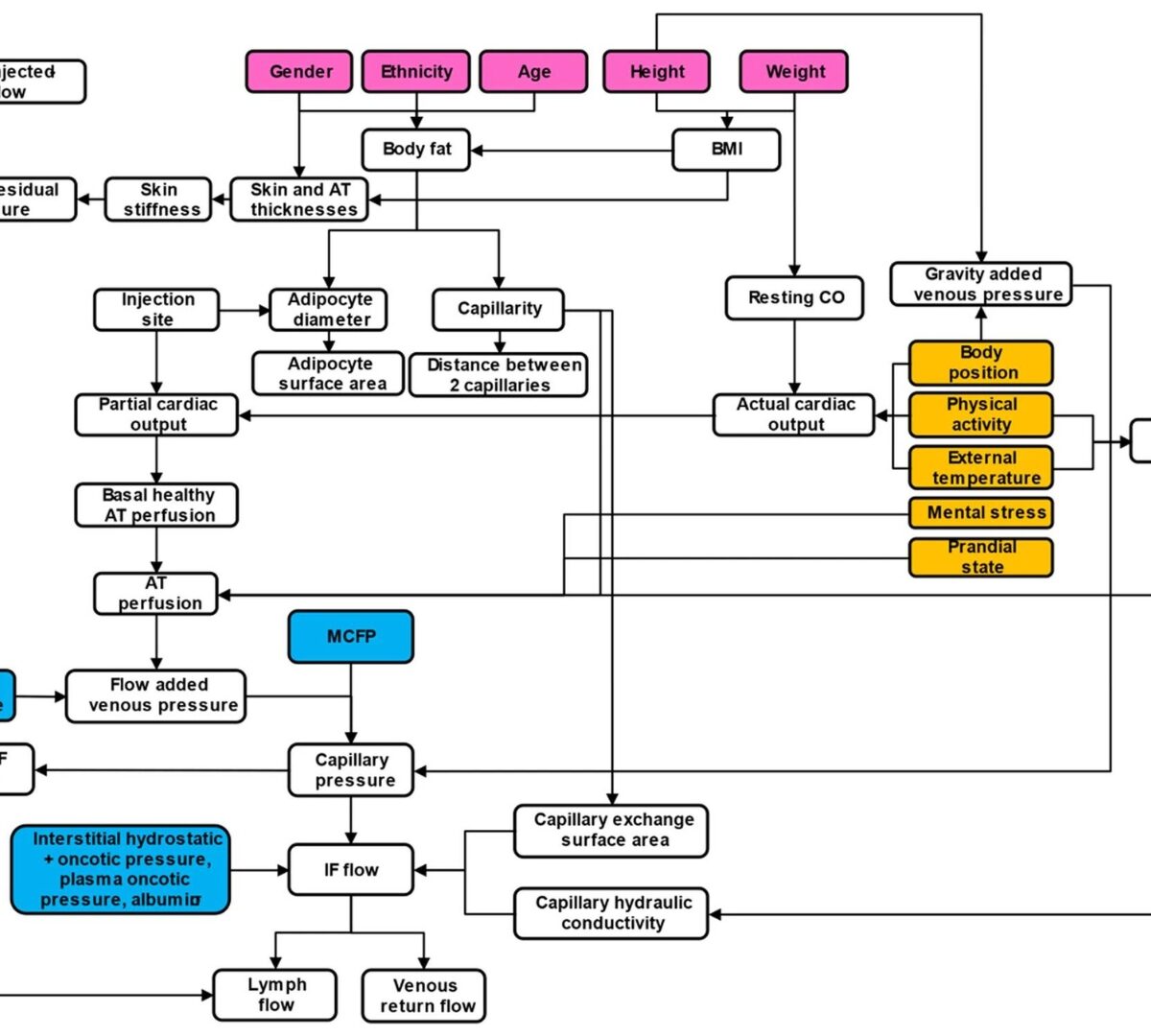
SubQ-Sim: A Subcutaneous Physiologically Based Biopharmaceutics Model. Part 1: The Injection and System Parameters
To construct a detailed mechanistic and physiologically based biopharmaceutics model capable of predicting 1) device-formulation-tissue interaction during the injection process and 2) binding, degradation, local distribution, diffusion, and drug absorption, following subcutaneous injection.
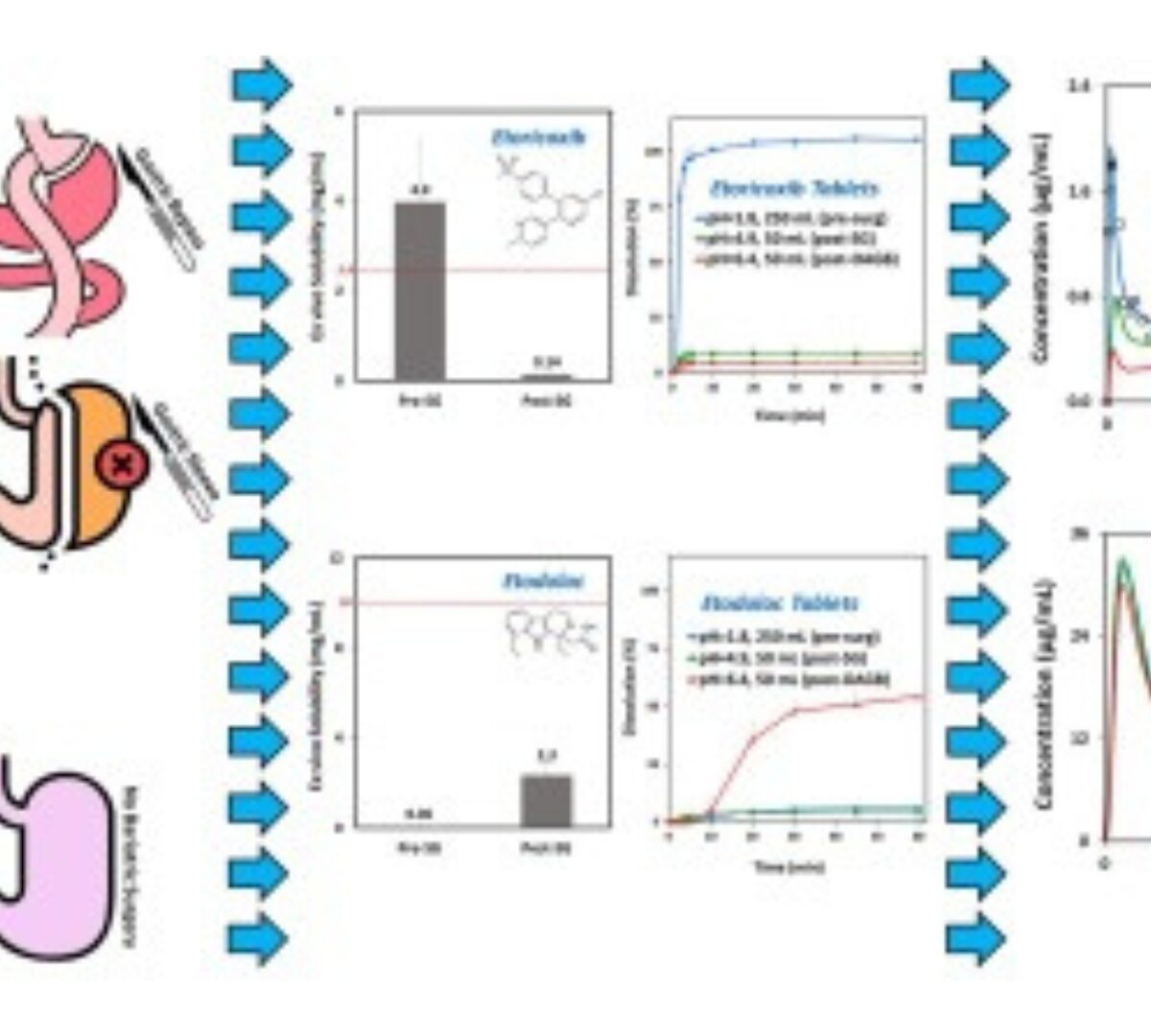
Selective COX-2 inhibitors after bariatric surgery: Celecoxib, etoricoxib and etodolac post-bariatric solubility/dissolution and pharmacokinetics
Anatomical/physiological gastrointestinal changes after bariatric surgery may influence the fate of orally administered drugs.

A robust, viable, and resource sparing HPLC-based logP method applied to common drugs
Reliable, experimentally determined partition coefficient P (logP) for most drugs are often unavailable in the literature. Many values are from in silico predictions and may not accurately reflect drug lipophilicity.

Halloysite nanotubes-cellulose ether based biocomposite matrix, a potential sustained release system for BCS class I drug verapamil hydrochloride: Compression characterization, in-vitro release kinetics, and in-vivo mechanistic physiologically based pharmacokinetic modeling studies
This study investigated the ability of natural nanotubular clay mineral (Halloysite) and cellulose ether based biocomposite matrix as a controlled release...
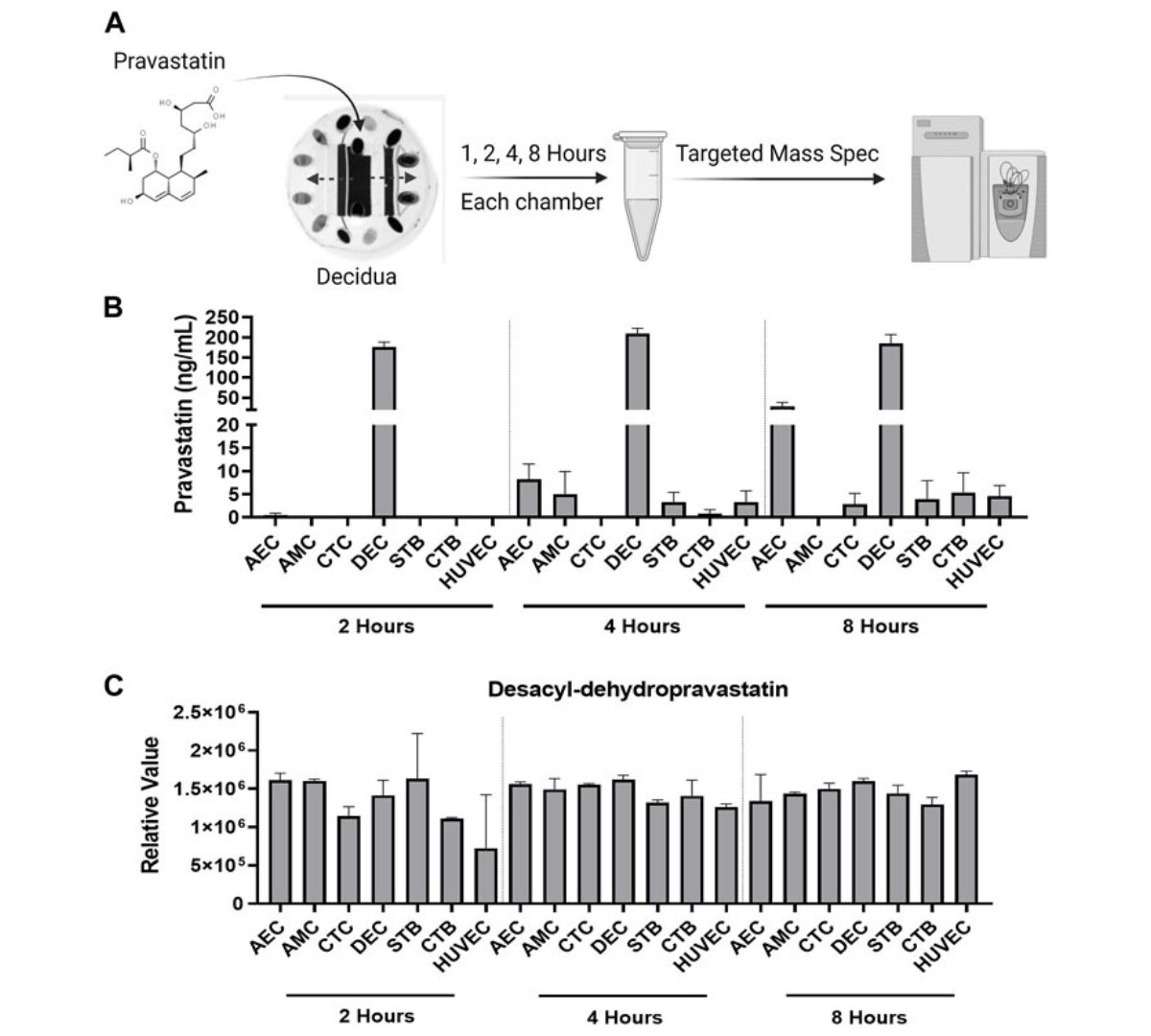
Microfluidic technology and simulation models in studying pharmacokinetics during pregnancy
Preterm birth rates and maternal and neonatal mortality remain concerning global health issues, necessitating improved strategies for testing therapeutic compounds during pregnancy.
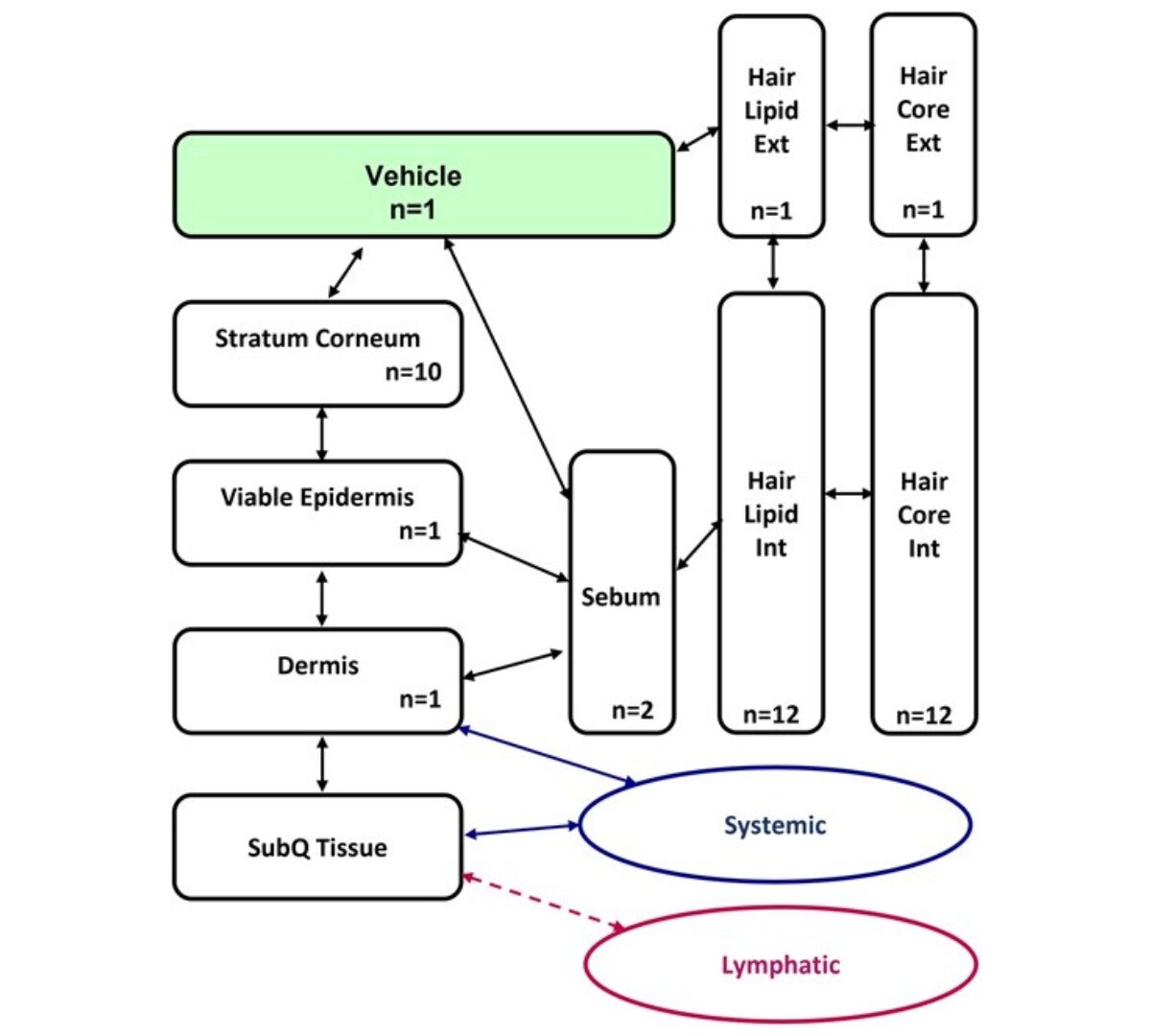
ADME characterization and PBK model development of 3 highly protein-bound UV filters through topical application
Estimating human exposure in the safety assessment of chemicals is crucial. Physiologically based kinetic (PBK) models which combine information on exposure, physiology, and chemical properties...

AIDD, an interactive AI-driven drug design system that uses molecular evolution and mechanistic pharmacokinetic simulation to optimize multiple property objectives simultaneously
Computer-aided drug design has advanced rapidly in recent years, and multiple instances of in silico designed molecules advancing to the clinic have...
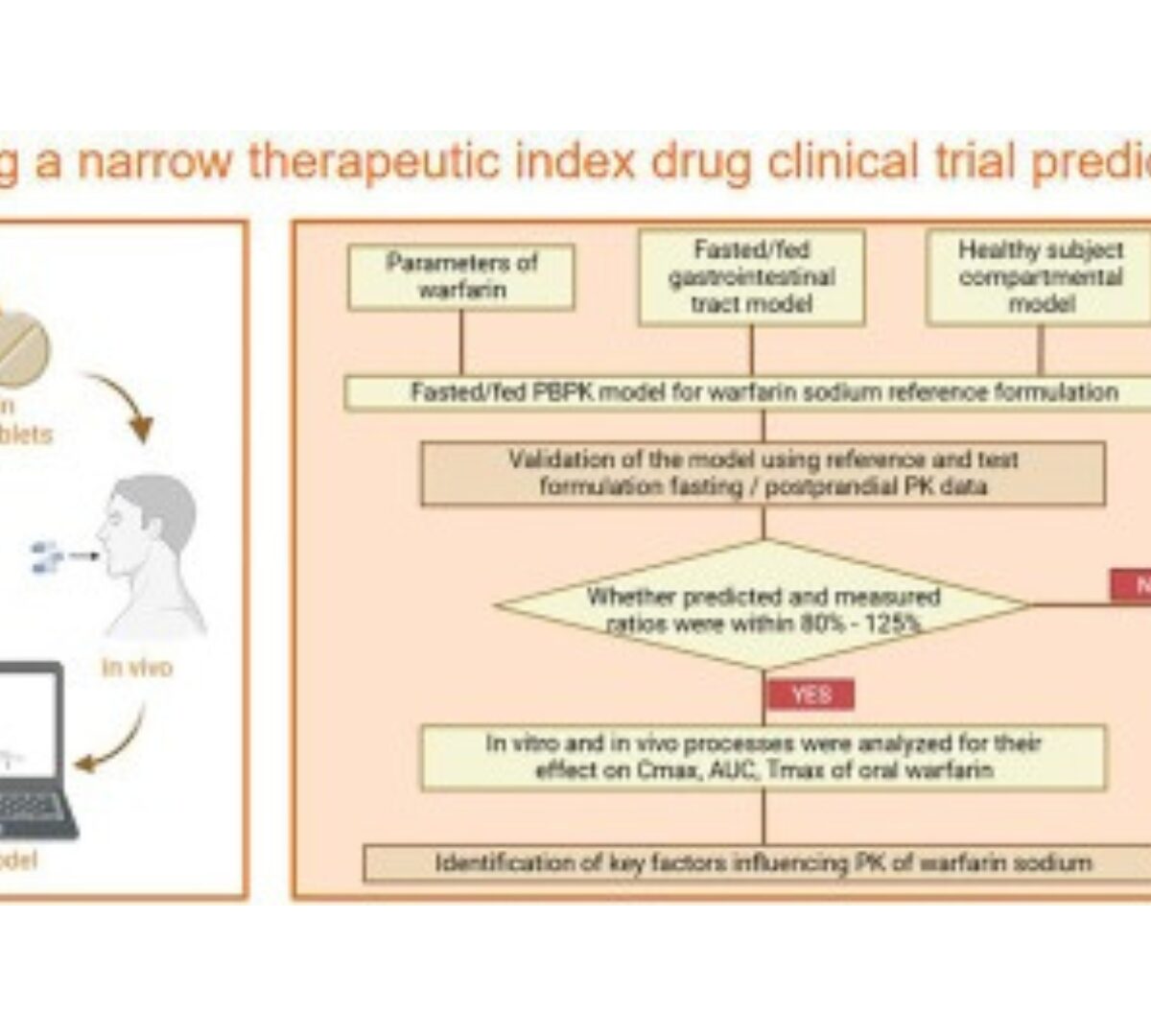
Predicting bioequivalence and developing dissolution bioequivalence safe space in vitro for warfarin using a Physiologically-Based pharmacokinetic absorption model
Bioequivalence (BE) studies support the approval and clinical use of both new and generic drug products.

Training the next generation of pharmacometric modelers: a multisector perspective
The current demand for pharmacometricians outmatches the supply provided by academic institutions and considerable investments are made to develop the competencies of these scientists on-the-job.

Physiologically based pharmacokinetic model combined with reverse dose method to study the nephrotoxic tolerance dose of tacrolimus
Nephrotoxicity is the most common side effect that severely limits the clinical application of tacrolimus (TAC), an immunosuppressive agent used in kidney transplant patients.