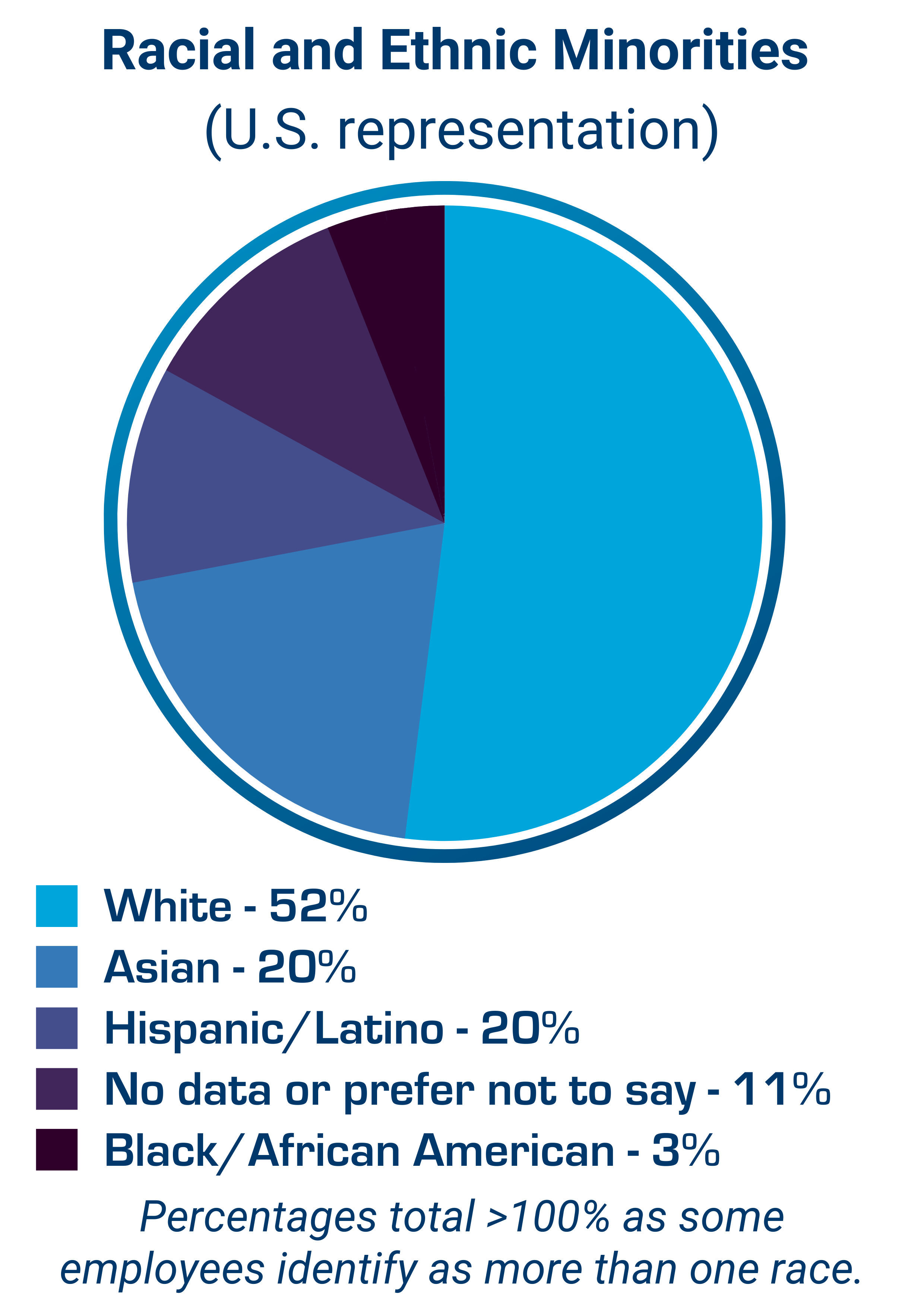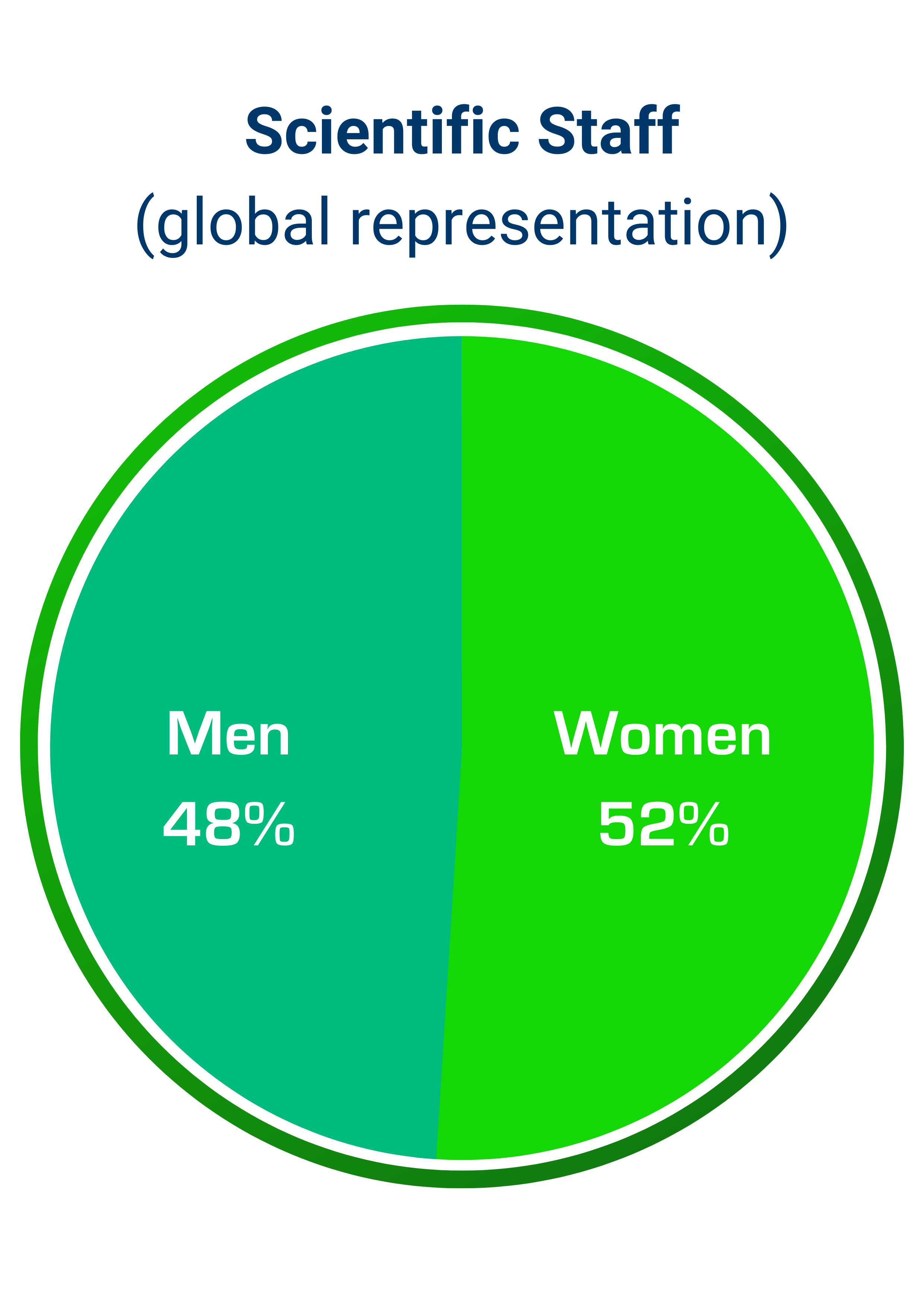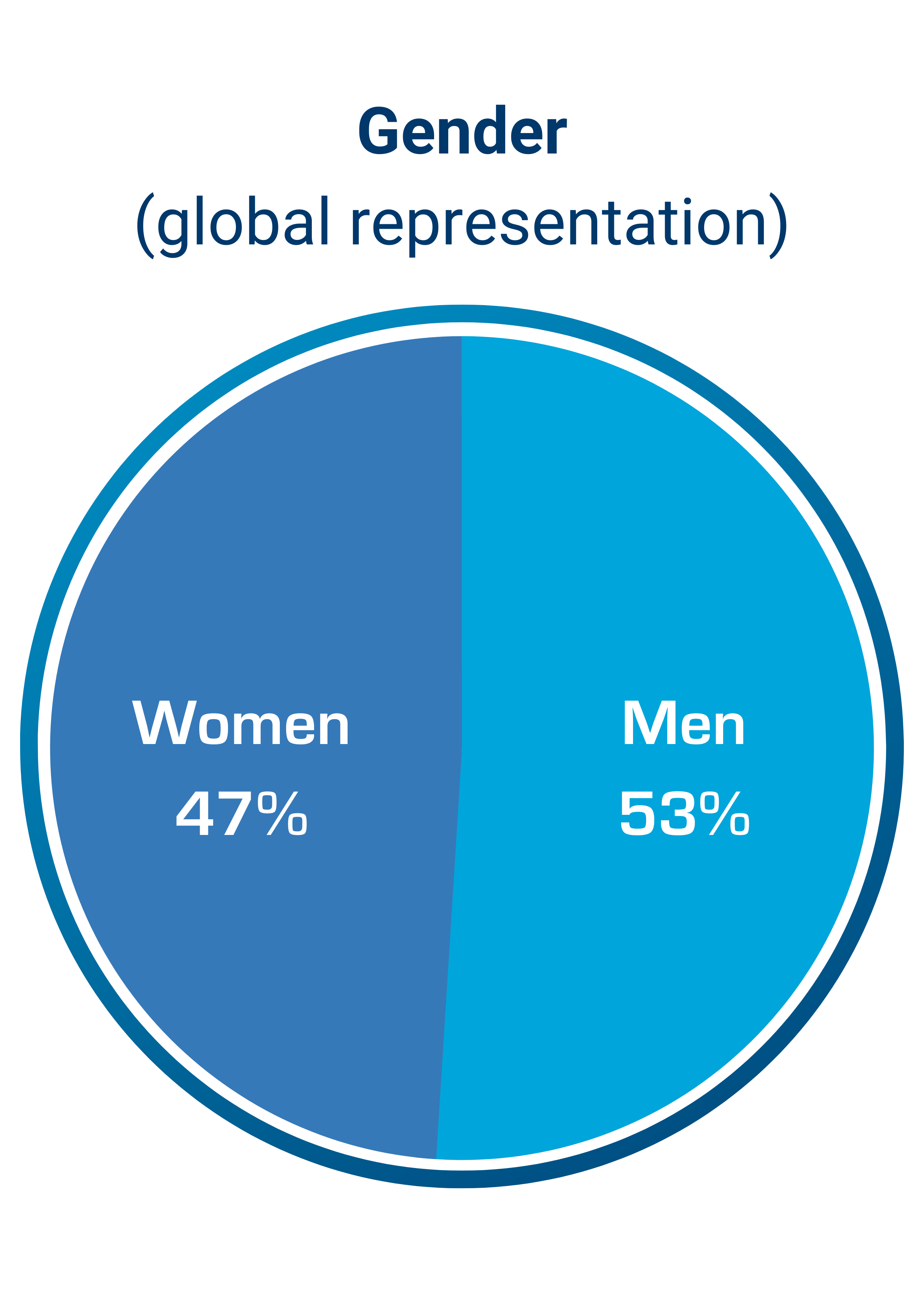
According to Pharmaceutical Research and Manufacturers of America, the research and development costs of bringing a new drug to market are estimated between $1 and $2.6 billion, at an average time of 12-15 years. These costs are inevitably borne by individuals, place a heavy burden on society and can have a profound impact on global health. Simulations Plus was founded and built on the concept that by integrating the complex scientific processes involved in drug discovery, development and approval into simulation and modeling software applications, thereby simulating laboratory experiments in silico, we enable researchers and regulators to perform sophisticated analyses of complex drug behaviors in humans and animals. The use of simulation and modeling applications allows our customers the opportunity to reduce unnecessary animal testing and lowers the risks of exposure to humans in clinical trials. Our software and consulting solutions, that bridge machine learning, physiologically based pharmacokinetics (PBPK), quantitative systems pharmacology/toxicology (QSP/QST), and population pharmacokinetic/pharmacodynamic (PK/PD) modeling approaches, support the companies and regulatory agencies involved in these life-saving endeavors.
To read more about our work in this area please see our website PBPK Modeling and Simulation
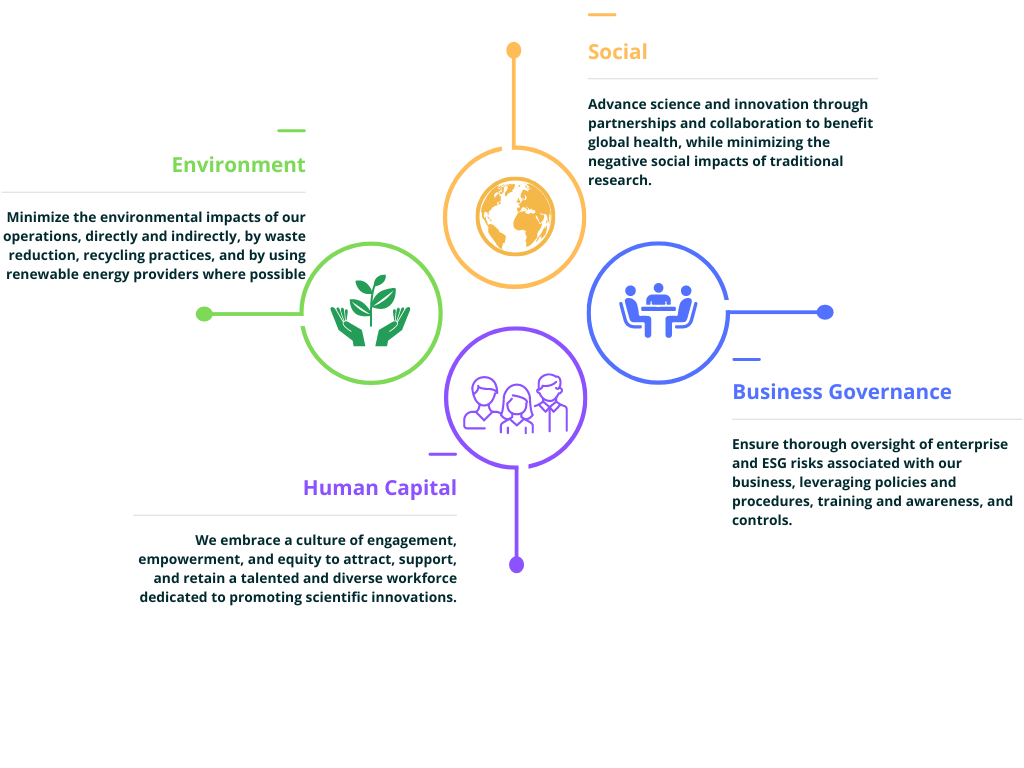
Corporate Philanthropy & Academic Support
We believe corporate philanthropy and academic support are both a privilege and a responsibility. We are privileged to be in a position where we can provide donations of time, financial support, and scientific expertise to academic institutions, research institutes, and to students. Our sense of responsibility is based on a belief that supporting academia and research is vital to the advancement of science, leads to greater collaboration between the academic and private sectors, and will unquestionably benefit society and the world, at large.
Our support for the academic community is broad and deep. We provide academics worldwide with free reference site licenses for nonprofit research and teaching, including free access to our software in university instruction, and substantial discounts on our training courses and workshops. We also offer a 90% discount on our software to government agencies and non-profit organizations. In recent years, we have sponsored several students with awards given by the Society of Toxicology.
We provide sponsorships to numerous conferences, symposia, and associations such as the American Conference on Pharmacometrics (ACoP), American Association of Pharmaceutical Scientists (AAPS), American Chemical Society (ACS), Controlled Release Society (CRS), Groupe de Métabolisme et Pharmacocinétique (GMP), and the Gordon Research Conferences. Individually, many of our employees, including our division presidents and scientists at all levels, volunteer their time to teach and mentor at universities and professional organizations, and frequently serve as peer reviewers for scientific journals.
At the local level, we promote a culture of volunteerism, and we offer our employees the flexibility they need to participate, from sponsoring and participating in charity golf tournaments to volunteering to serve hot meals to the disadvantaged. In recent years, we have joined the global GivingTuesday movement and donated food, clothing, and financial support to several organizations that serve those in need in our communities.
Our commitment to community, to education, and to gender equity can best be summarized by how we have, for more than a decade, funded a summer scholarship to Tech Trek, a one-week residential science, technology, engineering and math (STEM) camp founded and operated by the American Association of University Women (AAUW) that is designed to inspire young women to attend college, to major in STEM fields, and to pursue STEM careers. Our own female scientists, who are excellent role models for these young women, have volunteered their time to personally present our Tech Trek scholarship each year.