The RENAsym Consortium is a pre-competitive partnership between the DILIsym Services division of Simulations Plus and the pharmaceutical industry centered around the development of the RENAsym simulation tool. RENAsym is a mechanistic, mathematical model of drug-induced kidney injury, in the form of computational software applied to predict whether new drug candidates will cause renal injury signals in patients and to enhance the understanding of mechanisms that contribute to renal safety signals already observed in the clinic.
The consortium goals, much like those of the parallel DILI-sim Initiative, are to improve patient safety, reduce the need for animal testing, and reduce the costs and time necessary to develop new drugs. Now, one membership fee covers your membership in the DILI-sim Initiative and RENAsym Consortium, as well as access to deliverables from both!
The first official release of the RENAsym software was in October of 2021 and is now available. Several RENAsym posters showing applications and details have been presented and are available at our resource center. RENAsym version 1A includes rat and human parameter options, is multi-scale, and focuses on proximal tubule cell (PTC) injury. Some key elements and injury pathways are included such as the cellular energy balance; apoptosis, necrosis, and proliferation; mitochondrial dysfunction, toxicity, and DNA depletion; crystal nephropathy; and the inflammatory response (neutrophils, macrophages, dendritic cells, and mediators such as HMGB1, TNF-α, IL-1β, IL-6, IL-10, IL-18, and HGF).
Clinically relevant biomarkers are represented (e.g., alpha GST, KIM-1, GFR, creatinine, RBF). Finally, an integrated renal function model connects the cellular level damage with functional changes at the organ level, emphasizing hemodynamics, Na+, water reabsorption, and RAAS modulation. Kidney drug concentrations can be predicted with our flagship SLP product, GastroPlus®, and imported. A visual depiction of RENAsym v1A is below:
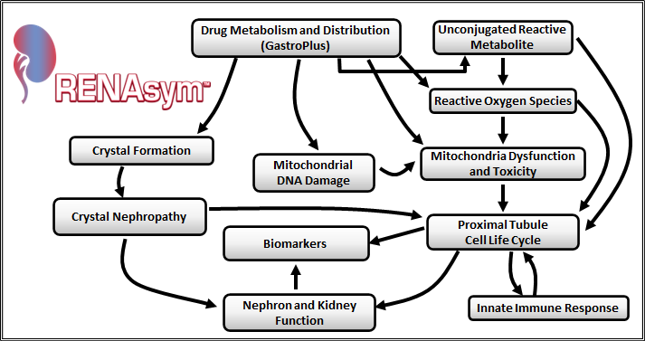
RENAsym v1A Summary Diagram
The first phase of funding for the development of RENAsym version 1A was provided by the NIH NIDDK SBIR small business grant process (click here for CIO policy related to NIH grants). In joining the RENAsym Consortium, members receive a prevailing voice in developing subsequent versions of RENAsym, ensuring future versions will be of maximum utility as it is applied within their respective organizations. Members also get the chance to hear and learn from one another at regular virtual and face-to-face meetings where scientific discussions centered around renal injury occur. Additionally, DILIsym Services has lined up a world-class Scientific Advisory Board to provide strategic guidance and direction for the consortium.
The DILIsym Services team leads the RENAsym Consortium development efforts. At the same time, the stellar Scientific Advisory Board is spearheaded by Dr. Paul B. Watkins, Director of the University of North Carolina Eshelman School of Pharmacy Institute for Drug Safety Sciences, located in the heart of Research Triangle Park, North Carolina. Several other esteemed academic experts in toxicology, modeling, and simulation, and renal injury and physiology have joined as well. Know more about our RENAsym Scientific Advisory Board

Dr. Paul B. Watkins, Howard Q. Ferguson Distinguished Professor, UNC Eshelman School of Pharmacy
RENAsym consortium members meet three to four times per year. Members receive updates on RENAsym development progress, propose new features, provide input on proposed additions, and engage in discussions regarding basic concepts and mechanisms related to kidney injury and function. Additional meetings are also held occasionally to discuss important scientific issues.
Commercial licenses outside of membership, as well as consulting engagements, are also available.
RENAsym® is computer software, namely, downloadable computer software for modeling kidney response to a drug or chemical; downloadable computer software for conducting simulations of kidney interactions with a drug or chemical; downloadable computer software for predicting pharmacokinetic, pharmacodynamic, and kidney responses to a drug or chemical.
To request a license for or evaluation of RENAsym®: https://www.simulations-plus.com/software-evaluation-request-form/
Members of the DILI-sim Initiative and RENAsym Consortium, which are covered under a joint membership agreement and annual fee, are provided the software deliverables below, along with training and access to the DSSI team for support, during their membership term:
- 2 global floating licenses to each software platform (DILIsym and RENAsym)
- Access to the high-performance grid license (HPGL) for both platforms upon release, which allows for scaling up simulation capabilities to an unlimited number of computational nodes.
- 10 free customized training hours per software tool
- Discounted or free attendance at workshops and courses
- A substantial discount on consulting services related to DILIsym and RENAsym
- Much, much more!
Simulations Plus Forms Scientific Advisory Board for RENAsym Consortium.