OBESITYsym, a quantitative systems pharmacology (QSP) model, was developed to help clients predict the efficacy of a weight loss therapy as well as its likelihood to cause nausea, one of the most commonly cited side effects of existing weight loss drugs.
Although the model had accurately predicted weight loss and nausea in other applications, it had not been used to simulate patient populations treated specifically for obesity.
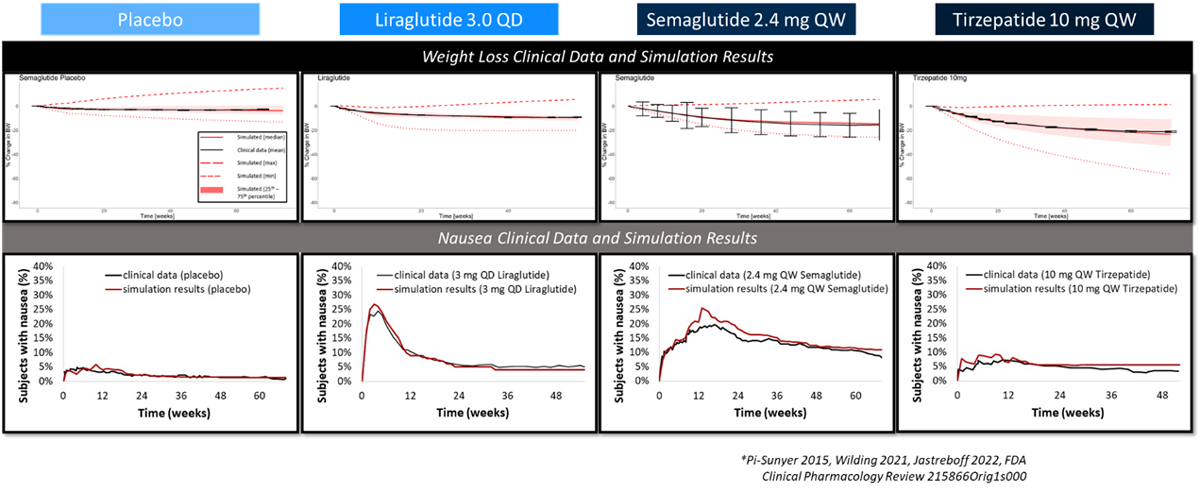
To ensure the model’s prediction capabilities were reliable, researchers decided to simulate treatment with three of the top weight loss drugs currently on the market: liraglutide, semaglutide and tirzepatide. A control group was also simulated.
The simulation for each drug included the recommended up-titration steps and associated doses. Each step was simulated as a four-week period (one week in the case of liraglutide). As each drug’s recommended up-titration process involved a different number of steps, the total treatment time for each varied between 56 weeks (liraglutide) and 72 weeks (tirzepatide). Each simulation also included a lifestyle intervention: a reduction of 50 to 200 kcal consumed per day and an increase of 25 minutes per day of physical activity.
In the case of each drug, there was excellent agreement between OBESITYsym predictions and the clinical data for weight loss and nausea. Researchers confirmed that the model was properly optimized to existing weight loss drugs and the model was capable of accurate, reliable predictions.
Learn more about how OBESITYsym can help optimize your drug for efficacy, adherence and market success.