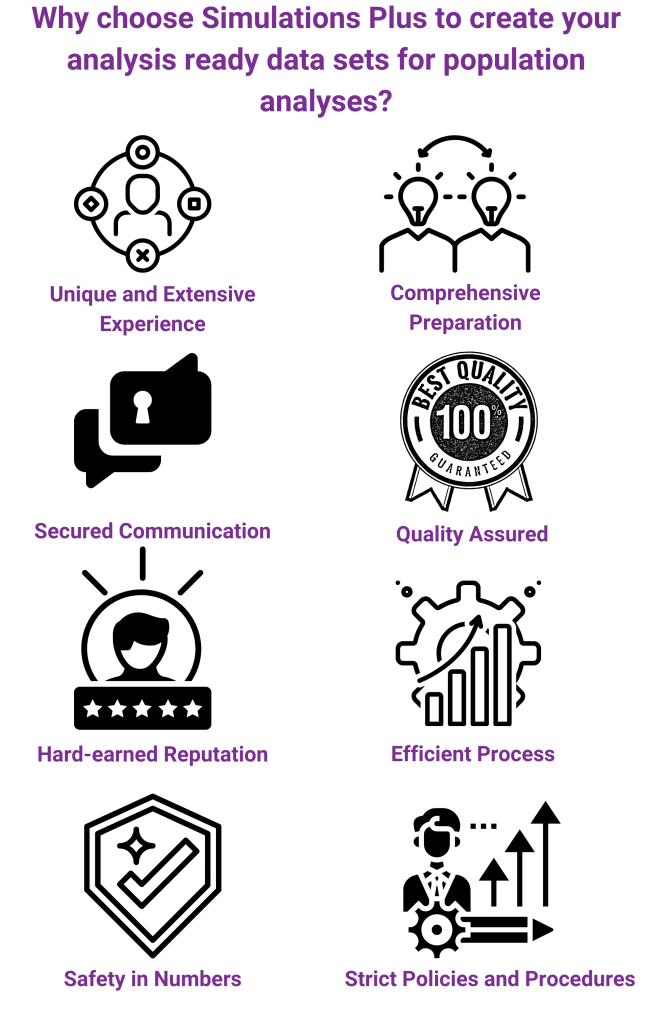
We take pride in our comprehensive preparation and efficient process for data assembly. Policies and procedures are in place to ensure quality of deliverables, and the data programmers have a toolkit full of macros, templates, and snippets to facilitate the conversion time and ensure the validation of the results. We undertake a complete review of the Investigator Brochure, protocols, case report forms, study reports, and literature to develop a thorough understanding of data assembly needs for pharmacokinetic and pharmacodynamic modeling. Throughout the project, we remain in constant contact with you and your team. We also use multiple tools to foster communication between scientists and data programmers.
In addition, we stay current with all data standards and are involved with a number of industry committees focused on efforts to streamline and harmonize data assembly and analyses-related activities.