Introduction
In part one of this complement blog a series, the complement cascade and its role in various diseases was introduced, as well as the potential for mechanistic modeling to support development of complement‑targeted therapeutics. COMPLEMENTsym, quantitative systems pharmacology (QSP) model of complement focused on the alternative and terminal pathways (AP, TP, respectively) was introduced.
In this post, we dive deeper into the publicly available data used to inform the model. We discuss in vitro data that can inform the model structure and particular parameter values. We also discuss data from human subjects that demonstrates the mechanistic representation of complement protein production, regulation, and clearance is consistent with circulating human complement analyte levels. We seek to address the following questions:
- What’s the nature and utility of the complement in vitro data?
- What’s the nature and utility of the complement in vivo data?
- How does this come together into a human representation?
- How can the modeling account for observed variability in human analytes?
Complement in vitro data
The complement cascade, including the various complement proteins, complexes, split products, and complement‑associated regulatory proteins, is well characterized1,2. To construct and parameterize a QSP model (initially focused on the alternative and terminal pathways), in vitro data were invaluable in establishing regulatory relationships, providing parameter values for known interactions and illuminating data gaps where parameter values were unknown but could be optimized to meet other constraints.
Multiple in vitro studies characterize binding kinetics of complement and complement‑associated regulatory proteins. For example, the association of C3b and Factor B (FB), as well as the dissociation of C3bB to its component entities, has been experimentally characterized using SPR biosensor analysis in multiple publications3–5. The resultant data were kinetically analyzed to identify association and dissociation rates, which could be applied directly in the model.
In another example, in vitro experiments measuring loss of complement C3 protein hemolytic activity over time were simulated using the same experimental concentrations of complement proteins 6. In these data, the addition of activating factors, Factor B and Factor D, caused a precipitous drop in C3 hemolytic activity as substrate was consumed; by contrast, the addition of regulatory proteins, Factor H and Factor I, dramatically mitigated the loss of C3 hemolytic activity. The inclusion of these various factors in the simulation not only serve to confirm their role in C3 disposition, but also serve to enforce that the quantitative relationships are accurately reproduced (Figure 1).
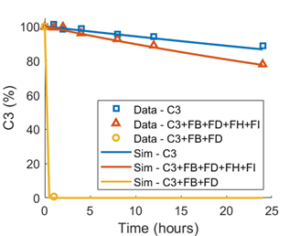
Figure 1. Simulated reproduction of in vitro data. Observed data6 map the spontaneous decay of C3 hemolytic activity in water (blue symbols), the rapid decay acceleration in the presence of Factor B (FB) and Factor D (FD) (yellow symbols), and the tempering of the rapid decay by addition of regulatory proteins, Factor H (FH) and Factor I (FI) (orange symbols). Simulated decay under the same conditions are shown in blue, yellow, and orange lines.
Notably, while the complement cascade is relatively rich in quantitative data characterizing the different constituents and their interactions, there are molecular entities which are difficult to quantify and for which no or minimal data were identified. For example, we know that C3 convertase (C3bBb) is converted to C5 convertase (C3bBbC3b) on binding of additional C3b molecules2; however, common constituents in the two entities pose challenges to experimentally characterize the rate of C5 convertase generation from C3 convertase. A further complexity to consider for incorporation in the model is that experimental data suggest that C3 convertase cleaves C5 albeit less potently than C5 convertase and vice versa7,8. In the absence of experimental data to define the conversion rate, model parameter values were optimized based on the ability to reproduce reported clinical levels of all analytes simultaneously. Importantly, the potential impact of the selected C3 to C5 convertase conversion rate may be interrogated as the parameter value was explicitly varied within the simulated population.
Complement in vivo data
Production and clearance
Soluble complement proteins are largely produced in the liver 9,10, although evidence for local production, particularly in disease conditions, has been reported 11,12. Metabolic labeling studies have been conducted for most of the primary complement proteins and their regulators to yield whole body production and clearance rates13–16. These data may be directly incorporated in a model.
Complement split products (e.g., C3a, C5a, Bb) are generated through complement activation, with increasing movement of complement proteins through the cascade2. Split products have been used as biomarkers of complement activation17. Interestingly, there are less publicly available data to characterize the half-lives of the split products. For example, the half-life of C5a has been estimated as one minute, based on the in vitro clearance of C5a following incubation with granulocytes or mononuclear cells18, as two to three minutes based on the clearance of injected C5a in rabbits19,20, or 30 minutes based on the in vitro degradation of C5a octapeptide by human plasma21. Similarly, the half-life for C3a has been estimated as four minutes based on the in vitro degradation of C3a octapeptide by human plasma21 and up to 161 minutes based on infusion and clearance of C3a desArg in normal healthy volunteers22. This latter data set brings in regulation of C3a and C5a by carboxypeptidases that cleave arginine from the carboxytermini, leading to C3a desArg and C5a desArg. The two catabolites are more stable, with the stability leveraged for assay detection23,24 but are functionally compromised. C3a desArg does not signal through the human C3a receptor25, while C5a desArg may express some activity depending on cell type26–29. Thus, inclusion of a longer half-life defined by a desArg catabolite may be appropriate since the catabolite is included in assay detection. However, modeling might consider decoupling the measured circulating value from receptor‑mediated activity based on the activity distinction between the proximal complement split product and the more stable catabolite. The appropriate choice of half-life may depend on modeling need and can be informed by ensuring compatibility with measured circulating analyte levels.
Measured analytes
A literature search was performed to identify available data reporting circulating levels of complement proteins, complement‑associated regulatory proteins, split products, and degradation products. There was considerable variability in how much data were identified for each analyte. For example, 17 publications were identified, in which human circulating C3 levels were reported for normal subjects. Twelve reported plasma levels, and assay types varied (e.g., ELISA, nephelometry). Study size ranged from nine individuals to 100. Despite these obvious sources of data variability, most of the studies (N=15) yielded similar concentrations of C3 in the normal healthy state, while two studies provided dramatically different values (Figure 2).
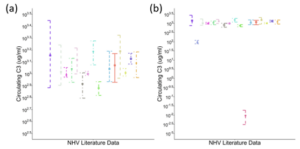
Figure 2. Circulating C3 from published literature. (a) N=15 sources yielding similar concentrations despite variability in study size, sample type, and assay type; (b) N=17 sources demonstrating two studies with distinct results.
In general, reported ranges for most analytes fell within an order of magnitude, despite differences in study size, sample type, and assay type. The exceptions were those for which limited data were available (e.g., iC3b).
Simulated individuals consistent with available data
COMPLEMENTsym represents AP and TP complement pathways in human circulation. As such, the simulated circulating levels of various complement proteins, regulatory proteins, split products, and degradation products are model outputs, to be compared against publicly available data. With an initial focus on the circulating compartment, simulated individuals are generated to be simultaneously consistent with data across all modeled analytes. As is often the case in modeling, we start with representation of a normal healthy state. We assume no inflammation in the normal healthy state, with complement levels being driven by steady state production, turnover, and the low‑level AP activation termed “tick‑over” that maintains the immune system’s ability to rapidly amplify a complement response if needed. An initial representation may use mean parameter values as inputs and compare the simulated individual against all comparator data (n=12 analytes), a subset of which is illustrated in Figure 3.
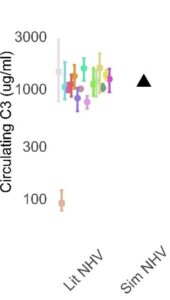
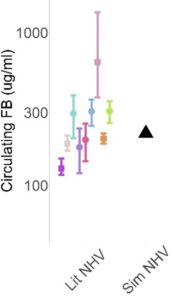
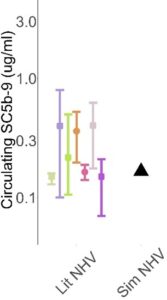
Figure 3. Illustrative comparison of a single simulated individual (black triangle) against published data (colored symbols) for C3, FB, and soluble C5b-9 (sC5b-9)
Once the normal healthy state is well established, we introduce perturbations consistent with our understanding of disease mechanisms to drive complement activation, resulting in circulating analyte levels consistent with disease populations of interest.
Alternate simulated individuals reproduce inter-patient variability
Within the QSP model, a simulated population reflects variability in the underlying biochemistry that manifests as variability in analyte concentrations. To learn more about simulated populations and the potential to leverage proprietary data to generate even higher fidelity populations, please reach out to our team.
Conclusion
COMPLEMENTsym curates publicly available in vitro and in vivo data to support generation of the model and qualification of simulated individuals and populations. In this blog post, we hope you have gained insight into the type of data that were available, challenges to interpreting the data, and how the data were used to generate simulated humans that reflect our current understanding of complement.
If this model would be useful to your work, please let us know.
References
- Risitano AM, Frieri C, Urciuoli E, Marano L. The complement alternative pathway in paroxysmal nocturnal hemoglobinuria: From a pathogenic mechanism to a therapeutic target. Immunol Rev. 2023;313(1):262-278. doi:10.1111/imr.13137
- Pouw RB, Ricklin D. Tipping the balance: intricate roles of the complement system in disease and therapy. Semin Immunopathol. 2021;43(6):757-771. doi:10.1007/s00281-021-00892-7
- Harris CL, Abbott RJM, Smith RA, Morgan BP, Lea SM. Molecular dissection of interactions between components of the alternative pathway of complement and decay accelerating factor (CD55). J Biol Chem. 2005;280(4):2569-2578. doi:10.1074/jbc.M410179200
- Hourcade DE, Mitchell LM. Access to the complement factor B scissile bond is facilitated by association of factor B with C3b protein. J Biol Chem. 2011;286(41):35725-35732. doi:10.1074/jbc.M111.263418
- Rooijakkers SHM, Wu J, Ruyken M, et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10(7):721-727. doi:10.1038/ni.1756
- Pangburn MK, Schreiber RD, Müller-Eberhard HJ. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981;154(3):856-867. doi:10.1084/jem.154.3.856
- Rawal N, Pangburn MK. C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J Biol Chem. 1998;273(27):16828-16835. doi:10.1074/jbc.273.27.16828
- Rawal N, Pangburn M. Formation of high-affinity C5 convertases of the alternative pathway of complement. J Immunol Baltim Md 1950. 2001;166(4):2635-2642. doi:10.4049/jimmunol.166.4.2635
- Alper CA, Johnson AM, Birtch AG, Moore FD. Human C’3: evidence for the liver as the primary site of synthesis. Science. 1969;163(3864):286-288. doi:10.1126/science.163.3864.286
- Morris KM, Aden DP, Knowles BB, Colten HR. Complement biosynthesis by the human hepatoma-derived cell line HepG2. J Clin Invest. 1982;70(4):906-913. doi:10.1172/jci110687
- Ahrenstedt O, Knutson L, Nilsson B, Nilsson-Ekdahl K, Odlind B, Hällgren R. Enhanced local production of complement components in the small intestines of patients with Crohn’s disease. N Engl J Med. 1990;322(19):1345-1349. doi:10.1056/NEJM199005103221903
- Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183-194. doi:10.1111/cei.12952
- Alper CA, Rosen FS. Alper CA, Rosen FS: Studies of the in vivo behavior of human C’3 in normal subjects and patients. J Clin Invest. 1967;46(12):2021-2034. doi:10.1172/JCI105691
- Charlesworth JA, Williams DG, Sherington E, Lachmann PJ, Peters DK. Metabolic studies of the third component of complement and the glycine-rich beta glycoprotein in patients with hypocomplementemia. J Clin Invest. 1974;53(6):1578-1587. doi:10.1172/JCI107708
- Charlesworth JA, Scott DM, Pussell BA, Peters DK. Metabolism of human beta 1H: studies in man and experimental animals. Clin Exp Immunol. 1979;38(3):397-404.
- Sissons JG, Liebowitch J, Amos N, Peters DK. Metabolism of the fifth component of complement, and its relation to metabolism of the third component, in patients with complement activation. J Clin Invest. 1977;59(4):704-715. doi:10.1172/JCI108689
- Kim AHJ, Strand V, Sen DP, et al. Association of Blood Concentrations of Complement Split Product iC3b and Serum C3 With Systemic Lupus Erythematosus Disease Activity. Arthritis Rheumatol Hoboken NJ. 2019;71(3):420-430. doi:10.1002/art.40747
- Oppermann M, Götze O. Plasma clearance of the human C5a anaphylatoxin by binding to leucocyte C5a receptors. Immunology. 1994;82(4):516-521.
- Webster RO, Larsen GL, Henson PM. In vivo clearance and tissue distribution of C5a and C5a des arginine complement fragments in rabbits. J Clin Invest. 1982;70(6):1177-1183. doi:10.1172/jci110716
- Weisdorf DJ, Hammerschmidt DE, Jacob HS, Craddock PR. Rapid in vivo clearance of C5ades arg: a possible protective mechanism against complement-mediated tissue injury. J Lab Clin Med. 1981;98(6):823-830.
- Campbell WD, Lazoura E, Okada N, Okada H. Inactivation of C3a and C5a Octapeptides by Carboxypeptidase R and Carboxypeptidase N. Microbiol Immunol. 2002;46(2):131-134. doi:10.1111/j.1348-0421.2002.tb02669.x
- Norda R, Schött U, Berséus O, et al. Complement activation products in liquid stored plasma and C3a kinetics after transfusion of autologous plasma. Vox Sang. 2012;102(2):125-133. doi:10.1111/j.1423-0410.2011.01522.x
- Burger R, Zilow G, Bader A, Friedlein A, Naser W. The C terminus of the anaphylatoxin C3a generated upon complement activation represents a neoantigenic determinant with diagnostic potential. J Immunol Baltim Md 1950. 1988;141(2):553-558.
- Oppermann M, Schulze M, Götze O. A sensitive enzyme immunoassay for the quantitation of human C5a/C5a(desArg) anaphylatoxin using a monoclonal antibody with specificity for a neoepitope. Complement Inflamm. 1991;8(1):13-24. doi:10.1159/000463173
- Wilken HC, Götze O, Werfel T, Zwirner J. C3a(desArg) does not bind to and signal through the human C3a receptor. Immunol Lett. 1999;67(2):141-145. doi:10.1016/s0165-2478(99)00002-4
- Werfel T, Oppermann M, Butterfield JH, et al. The human mast cell line HMC-1 expresses C5a receptors and responds to C5a but not to C5a(desArg). Scand J Immunol. 1996;44(1):30-36. doi:10.1046/j.1365-3083.1996.d01-272.x
- Schulman ES, Post TJ, Henson PM, Giclas PC. Differential effects of the complement peptides, C5a and C5a des Arg on human basophil and lung mast cell histamine release. J Clin Invest. 1988;81(3):918-923. doi:10.1172/JCI113403
- Gerard C, Chenoweth DE, Hugli TE. Response of human neutrophils to C5a: a role for the oligosaccharide moiety of human C5ades Arg-74 but not of C5a in biologic activity. J Immunol Baltim Md 1950. 1981;127(5):1978-1982.
- Bürgi B, Brunner T, Dahinden CA. The degradation product of the C5a anaphylatoxin C5adesarg retains basophil-activating properties. Eur J Immunol. 1994;24(7):1583-1589. doi:10.1002/eji.1830240720
